Research Article | DOI: https://doi.org/CCSRR-RA-26-47
Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients: a Narrative Review of Early and Long-Term Outcomes
Abstract
For patients with severe aortic stenosis who are otherwise at low surgical risk, deciding between transcatheter aortic valve replacement (TAVR) and surgical aortic valve replacement (SAVR) isn’t straightforward. Trials like PARTNER 3 and Evolut Low Risk, supported by solid long-term data, suggest that TAVR is an excellent alternative. It’s less invasive, offers quicker recovery, matches or even surpasses surgery in survival rates, and lowers stroke risk for 5 to 7 years. But TAVR isn’t without downsides—it carries a higher risk of needing a permanent pacemaker and often leads to paravalvular leaks. Meanwhile, SAVR patients more frequently experience new atrial fibrillation and acute kidney injury. Ultimately, the choice depends on which risks and benefits matter most to the individual.
This review shows us different trails, registries and meta-analyses that are done recently to compare early, mid-term and long-term outcomes of TAVR vs SAVR in the patients that have a low risk of surgery, especially in young people with bicuspid aortic valves. After TAVR the chances of having complications like major bleeding and acute kidney injury are low, TAVR also provide early recovery. For younger, low-risk patients and those with bicuspid valves, the latest three-year data looks promising but isn’t complete yet. Because of this, current guidelines stress the importance of personalized decisions made by a heart team, focusing on long-term care instead of automatically choosing “TAVR first” or “surgery first” for everyone.
Introduction:
In the modern cardiology world, severe aortic stenosis (AS) has become a great valve problem. It is not just affecting the old people but nowadays affecting young fitter people. They go through the risk scores such as STS-PROM which is a risk score for mortality for 30-day post op.(1) They also go through another risk score that is EuroSCORE,(2) this starts a debate between whether transcatheter aortic valve replacement (TAVR) procedure should be opted for the patient or the surgical aortic valve replacement (SAVR) procedure.(3) striking not just the very old and frail but increasingly fitter patients who sail through risk scores like STS-PROM (<4>
Epidemiology of Aortic Stenosis (AS)
Aortic stenosis is caused by thickening and calcification, which is more common among individuals under the age of 65, at a rate of 1.2%. The prevalence of severe AS is 0.2% among individuals aged 50 to 59 years, 1.3% among those aged 60 to 69 years, and almost 10% among those aged 80 to 89 years.(1) There is an incidence of 5 new cases per 1,000 people every year, with an average age of 60.(12) Men are slightly more affected than women,(13) with an incidence of around 52 cases per 100,000 people every year. In the US, there are approximately 100,000 annual interventions, with 40 to 50% of low-risk cases (STS < 4>
TAVR's Evolution from Last Resort to Frontline
In 2002 for the patients who are unable to be operated on for severe AS they have gone TAVR procedure, which has been life saving for them. (16)TAVR procedure’s noninferiority has been proven by High-risk PARTNER 1A/1B (2011) compared to SAVR procedure.(17) Intermediate-risk PARTNER 2A/SURTAVI (2016-17) closed gaps,(17) then low-risk patients according to PARTNER 3 trail (2019; n=1,000, mean age of 73, STS 1.9%) showed TAVR superiority on 1 year.(18) Problems like death or stroke or rehospitalization (8.5% vs 15.1% SAVR), Evolut Low Risk (2019; n=1,467, age 75, STS 1.8%) became noninferiority, and on 2-year death or disabling stroke (5.3% vs 6.7%) became less but Pacemakers rose up (7-19% TAVR vs 5-7% SAVR),(18) but bleeding or AKI or AF plummeted sans bypass. Registries like STS/ACC TVT confirmed low-risk TAVR is more than 70% now in US by 2024.(19)
Meta-analyses
It confirm these findings, with Kolte et al. reporting a relative risk of approximately 0.53 for risk of death at 30 days with TAVR versus SAVR using a pool of over 20,000 patients, with no clear distinction between the curves at 2 years.(20) FU and associates also reported an incidence of about half that of acute kidney injury and major bleeding complications with TAVR in low- to intermediate-risk patients. However, significant trade-offs continue to exist, including new onset of conduction abnormalities requiring pacemaker placement in about 10-25% of TAVR recipients and mild PVL in about 20-30%, (21) plus an inherent and as-yet-undefined risk of prosthesis durability at >10 years in patients under the age of 70 potentially facing an expected survival of 15-20 years following treatment. Bicuspid valves continue to introduce an added level of complexity, with increased risk of prosthesis-patient mismatch and PVL, and TAVR potentially complicating coronary re-entry at some point down the road.
Low-Risk Cohort Spotlight
Low-risk patients are commonly characterized by an STS-PROM or EuroSCORE II of < 4>
Methods:
The literature review applies a focused evidence synthesis based on randomized controlled trials (RCTs), prospective registries, as well as the most recent meta-analyses available to compare transcatheter aortic valve replacement (TAVR) with surgical aortic valve replacement (SAVR) among patients with low to intermediate risk who have severe aortic stenosis (AS). The literature was selected based on its rigor with a focus on multicenter randomized studies, blind endpoints, as well as longer-term follow-up where possible—in this case, studies with a Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) of below 4% who reflect low-risk patients.(23)
Primary Randomized Controlled trials
In the PARTNER 3 trial (NCT02675114), published in the New England Journal of Medicine (Mack et al., 2019), 1,000 low-risk patients (mean STS score of 1.9%) underwent randomization in a 1:1 manner to undergo transfemoral TAVR with the SAPIEN 3 device or SAVR. The primary endpoint was the composite of death, stroke, or rehospitalization at 1 year, with long-term follow-up at 7 years demonstrating no differences in mortality or valve survival. In the Evolut Low Risk trial (NCT02701283), published in New England Journal of Medicine (Popma et al., 2019), 1,467 patients (mean STS of 1.9%) underwent randomization to receive self-expanding Evolut R/Pro valves or SAVR and met the predefined criteria of non-inferiority of TAVR to SAVR in the composite of death or disabling stroke at 2 years, which persisted at 5-year follow-up. In addition to the above findings, this long-term follow-up proved TAVR superior in terms of hemodynamics and associated with significantly higher rates of permanent pacemaker implantation. In the NOTION trial (Thyregod et al., 2024), 280 lower-risk patients with 82% STS <4>
Early 30days Outcome
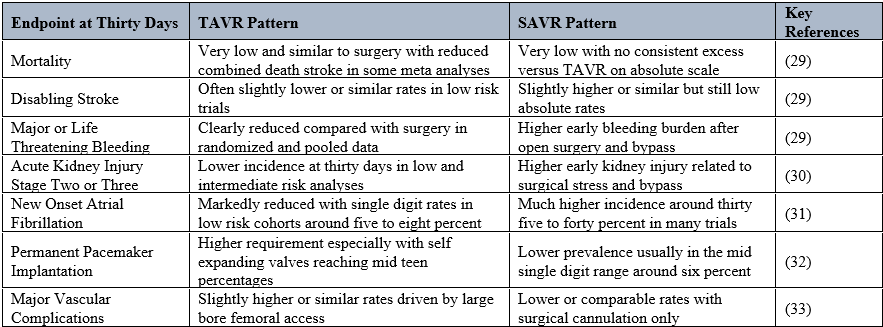
Contemporary low risk trial results reveal a. very low. thirty-day mortality and stroke with TAVR although comparable to surgical AVR as well (TAVR had more permanent pacemakers, but less vascular complication).(25–27) The same trends have been identified in individual RCT's and in recent meta-analyses on low/intermediate risk patients.(25–27)
Results from the PARTNER 3 study showed that the SAPIEN 3 transcatheter valve had less major neurological complications (such as stroke and atrial fibrillation) during the first month than surgery.(28) The frequency of strokes was approximately 0.5% within 30 days and 2.5?ter surgery.(28) Atrial fibrillation developed in approximately 5% of patients undergoing TAVR compared to approximately 40% undergoing surgical AVR.(28)
The composite endpoint of death, stroke, or hospitalization also remained lower in the transcatheter cohort (95.7% vs. 93.4%) at one year post TAVR/surgery. There was no significant difference in the rates of major vascular complications (0.4% vs. 0.4%) or the need for a permanent pacemaker (0.5% vs. 5%) following TAVR with a balloon expandable valve. (28)Therefore, the overall safety profile of transcatheter therapy in this low-risk patient population was beneficial during the first year. Stroke, bleeding, and atrial fibrillation were the most significant contributors to this benefit as opposed to differences in mortality alone.(28)
In the Evolut Low Risk trial, self-expanding valves demonstrated non-inferiority for death or disabling stroke at two years (24 months) and promising early safety data for renal injury, bleeding and AF.
At 30 days, life-threatening/disabling bleeding was < 2> 7?ter surgical, while acute renal injury/AF were consistently lower in frequency following less invasive procedures.(17)
These benefits come at the cost of increased likelihood of permanent pacemaker/procedure implantations (approximately 17% of patients required a device within 30 days of Evolut transcatheter therapy, as opposed to about 6% following surgical) and slightly more paravalvular regurgitation. Major vascular complications at 30 days using these two approaches were similar, indicating that modern access techniques can reduce large vessel injury even when large bore catheters are used.(17)
Meta-analysis of randomised controlled trials of low-risk patients undergoing percutaneous valve implantation report significantly lower rates of death or stroke at 30 days following percutaneous valve implantation when compared to surgical implantations (rate ratio approximately 0.7). Less early bleeding, new onset atrial fibrillation, acute renal failure and prosthetic heart valve mismatch, but greater numbers of permanent pacemakers, reintervention and paravalvular leak are complications associated with transcatheter procedures as opposed to surgical procedures. (25,27)
A longitudinal meta-analysis, which included ten randomised controlled trials involving over 9,000 patients, supports the notion that most differentials between transcatheter valve replacement and surgical valve replacement exist during the first year after procedure in terms of combined death or stroke as well as safety endpoints. The difference diminishes with longer follow up, indicating that 30-day outcomes are primarily determined by the degree of invasiveness and injury incurred by procedure.(25)
Various systematic studies over the last few years have recognized in detail 30, day safety outcomes of transcatheter replacement in low to intermediate, risk patient cohorts, most recently employing the Valve Research Consortium criteria for the description of the safety outcomes. (25–27) The data of these studies demonstrate that transcatheter replacement is linked to a markedly reduced occurrence of life, threatening or major bleeding events. In addition, the rates of stage 2, 3 acute kidney injury are lower. A very small number of patients who underwent transcatheter replacement developed atrial fibrillation early in the post, procedure period, as compared to surgical replacements. Correspondingly, low mortality as well as disabling stroke rates have been reported for both approaches. (25,26)
Both methods still carry risks, as evidenced by the odds of major vascular complications and of getting a permanent pacemaker within thirty days of the procedure, which are higher after transcatheter valve replacement as compared to surgical valve replacement. Hence, when deciding between transcatheter and surgical approaches to valvular disease, it is advisable to weigh factors such as patient's age, physique, risk of developing conduction abnormalities, and vascular status prior to making a final decision on therapy.(25–27)
Mid to long term outcomes:
TAVR has been shown through long-term studies to be a viable alternative to surgical aortic valve replacement (SAVR) up until approximately 7 - 10 years post replacement and provides more favorable hemodynamic results (greater than that of SAVR), less structural valve deterioration (SVD) in some cohorts and long-term gains in terms of quality of life. Therefore, TAVR can be considered as an option for low-risk patients with symptomatic, severe aortic stenosis especially when the life expectancy aligns with the currently known durability of TAVR.
The Evolut Low Risk Trial (ELRCT) also evaluated patients who were at low risk for undergoing TAVR (<75>
In patients <75 p=0.241).>
The hemodynamic performance of TAVR during this period clearly demonstrates the benefit of taking TAVR. At 3 years, TAVR patients had an average aortic gradient that was lower than that of SAVR patients (9.7 mmHg vs 12.9 mmHg; P<0>
From a safety perspective, the price to be paid for the hemodynamic advantages of TAVR is minimal. The rate of new permanent pacemaker implantation is consistently higher for TAVR with self-expanding valves; however, trends in mortality and stroke are generally in favor of SAVR or match surgery trends. Although patency rates of TAVR are slightly more frequent, moderate or severe patency of TAVR has not been reported as occurring with commercially available technology.
7 Years – Equivalent Clinical Durability
The PARTNER 3 trial represents the most robust data to date regarding the performance of balloon-expandable valves in low-risk populations. The incidence of death, stroke and rehospitalisation at 7 years was similar for both TAVR (34.6%) and SAVR (37.2%) patients (difference −2.6 percentage points; 95% CI: −9.0 to 3.7). Additionally, the incidence of death from any cause was 14.2% for TAVR patients and 16.6% for SAVR patients, while the incidence of stroke was also similar (TAVR 6.0% vs SAVR 6.4%). Therefore, the data indicate that TAVR has not shown greater disadvantages beyond the first seven years when compared to SAVR procedures.(35,36)
TAVR efficacy over five years has been demonstrated to be in line with that of SAVR by self-expanding TAVRs through the Evolut Low Risk Trial with an overall mortality or disabling stroke rate of 15.5% TAVR and 16.4% SAVR. This provides further evidence for long-term durability of both TAVR and SAVR, with similar overall mortality rates after five years (around 10%). In the case of TAVR, the hemodynamic advantage of a supra-annular valve compared with surgical valves continues to remain unchanged after midterm follow-up. TAVR provides a more favorable hemodynamic model in general than does the optimal model. TAVR continues to have more favorable gradient and effective orifice area characteristics when compared with surgical bioprosthesis valves; comparative analyses do not show any statistically significant differences between early valve-related complications (EVA), rates of valve thrombosis, or rehospitalization or reintervention rates for TAVR vs. SAVR. The PARTNER 3 study observed that after seven years, there were between 10% of rehospitalizations due to valve-related complications for TAVR; and between 7% and 8% for SAVR, well within the overlapping confidence intervals for both groups; while reintervention rates were reported as 2%3% at five years for both TAVR and SAVR in the Evolut Low Risk Trial.(35)
The primary factor distinguishing long-term outcomes is structural valve deterioration. While TAVR had a significantly lower rate of SVD than SAVR at 8 years (13.9% vs 28.3%, p=0.0017), the overall rate of bioprosthetic valve failure was similar for both groups (8.7% for TAVR and 10.5% for SAVR). The results suggest that early generation self-expanding TAVR valves are likely to remain as durable as surgical bioprosthetic valves for patients of this age/risk profile, and may be more resistant to hemodynamic impairment than surgical bioprosthetic valves. However, experts caution against assuming that the true burden of SVD for younger low-risk patients has been accurately characterized, and recommend further follow-up before making generalizations about the 10-year durability of TAVR valves in patients in their 60s.(36)
Echocardiography performed on patients in these long-term studies showed that TAVR valves have lower transvalvular gradients and greater effective orifice areas than surgical valves, which is consistent with the design of the supra-annular TAVR valve. However, PVL remains slightly more prevalent in patients undergoing TAVR, and the long-term consequences of chronic PVL, left ventricular remodeling and heart failure is still being clarified. The rate of reintervention over the course of follow-up was low and similar for both groups, and valve-in-valve TAVR is an option for patients who experience bioprosthetic valve failure.(37)
Compared with TAVR, traditional surgical methods such as SAVR (Surgical Aortic Valve Replacement) provide more difficult recovery periods due to the peri-operative trauma associated with open-heart surgeries.
Patients will achieve significantly increased KCCQ (Kansas City Cardiomyopathy Questionnaire) scores approximately 30 days after their TAVR procedure, representing about a 20–25-point increase from their KCCQ baseline level.
Furthermore, when comparing quality of life and functional status between TAVR and SAVR, both groups maintain significant long-term benefits with very stable improvement for many years after the procedure. The PARTNER Trial 3 study presented these findings, showing that both TAVR and SAVR patients maintained substantial and sustained increases in functional status over many years, and the majority of these patients remain in NYHA Class I or II (New York Heart Association Functional Classification System).(38)
Subgroups
Younger patients and patients with Bicuspid Aortic Valves (<75>
The Modine/Evolut study of younger patients (<75>
The Evolut Low Risk bicuspid study enrolled 150 patients who were considered to be at low risk for surgery, i.e., they had a mean age of 70 years, and their STS PROM (Society for Thoracic Surgery Predicted Risk of Mortality) was 1.3%, and they received treatment with Evolut R / PRO self-expanding valves. Study results have shown very low combined event rates of death and disabling stroke at 3 years (approximately 4%). The hemodynamics of all patients in this study remained excellent with sustained valve gradients and the absence of moderate or severe paravalvular leakage (PVL) through the 2- and 3-year follow-up periods. The majority of patients had no PVL or trace amounts of PVL.(40)
Studies have documented that cardiovascular conduction abnormalities are a significant complication associated with TAVR, leading to a new PPM (permanent pacemaker) implant in approximately 19% of TAVR patients by 3 years. Evidence for the long-term structural performance of TAVR valves in the setting of bicuspid valves and the frequency of reinterventions after 3 to 5 years is limited. Consequently, although the AHA/ACC guidelines still support the use of surgical aortic valve replacement (SAVR) for younger patients with bicuspid valves, this recommendation is more strongly supported when these patients also have associated aortopathy.
Subgroup pattern versus overall trial
The primary composite endpoint for death/disabling stroke shows similar hazard ratios (HRs) between younger patients/bicuspid patients, indicating TAVR is non-inferior to SAVR with respect to major stroke/survivability, across low-risk trial groups.
Disabling stroke numbers favour TAVR, but new pacemaker insertion, prosthesis-patient mismatch vs anatomic complexity shift the risk-benefit ratio, therefore should be more carefully considered for patients who will likely live longer than the first prosthesis.
Long-term coronary access
In younger patients, long-term coronary access is an important concern, particularly for those who will require multiple future PCI (percutaneous coronary interventions). Tall-frame, supra-annular THV (transcatheter heart valve) devices implanted high within the left ventricle or in small sinuses may obstruct or greatly reduce the ability for selective coronary cannulation (via the ostia), making this increasingly difficult following TAVR (transcatheter aortic valve replacement) performed on a prior TAVR patient.(41)
Patients with complex or extensive coronary artery disease or whose future interventions are anticipated to be via coronary artery bypass graft (CABG) or percutaneous coronary intervention (PCI) should consider either a surgical valve replacement (SAVR) with a bioprosthetic heart valve or the use of TAVR depending on the time frame for intervention.
Coronary Access Preservation for Long-Term Success: To preserve long-term access to coronary arteries, precise pre-procedural CT planning, frame design with optimal commissural alignment and avoidance of higher-than-necessary placements of valve frames should be considered. For patients who have very complex coronary artery disease and/or very diffuse disease or who are likely to have future CABG or PCI, surgical valve replacement with a bioprosthesis may provide superior, more reliable, and more predictable access to coronary arteries than a TAVR-first approach.(41)
Valve-in-Valve and Lifetime Strategy
As TAVR continues to expand its utilization to younger patients with low surgical risk, many of these patients will experience the need for reintervention in the form of valve-in-valve TAVR rather than open surgical reoperation. Although redo-TAVR is safe and feasible in most cases, it may increase a patient's prosthetic valve mismatch and further limit access to the coronary arteries, especially if the original prosthesis has been implanted into the aortic root in a restrictive manner.(42)
The current best practices of managing patients throughout their lives are to plan for the patient when they have a first procedure by determining the best prosthetic device and the best way to implant the prosthesis, so that there is a large enough effective orifice area, that there is a minimum of severe prosthesis patiënt size mismatches and that the prosthesis allows for coronary access in the event that the pacient needs a TAVR-in-TAVR. For very young low-risk patients, patients who have a bicuspid valve, patients who have small annuli, and patients who have a complex coronary anatomy, it may still be safer to perform a SAVR procedure first (with the possibility of later doing a TAVR-in-SAVR) than to do a TAVR procedure first, even when the early results of TAVR appear to be very good.(43)
Discussions:
Though TAVR has several advantages compared to SAVR, there are trade-offs associated with PPM, PVL, and long-term durability when performed in younger patients. ACC/AHA and ESC/EACTS guidelines recommend that each patient undergo a heart team evaluation prior to undergoing TAVR and currently, there are still knowledge gaps present in the literature regarding bicuspid valves greater than 10 years and next-generation devices. The majority of patients undergoing low-risk surgical aortic valve replacements are currently being treated with TAVR (over 70%) based on robust pre-inscriptive discussions and the results from ongoing randomized controlled trials (RCTs) will continue to provide more guidance moving forward. (28,44)
TAVR has demonstrated numerous short-term benefits associated with lower overall mortality at 30 days post-procedure, decreased hospital readmissions, and rapid recovery compared to SAVR in low-risk populations; however, there are concerns associated with higher rates of PPM (up to 15-20%) and PVL (up to 15-20%), particularly due to conduction disturbances and long-term risk for heart failure. The SAVR approach to replacement provides improved durability in patients <65>
Comparison of Guidelines
In their recent guidelines, ACC/AHA prefer to use surgical aortic valve replacements (SAVR) for patients younger than 65 years and for those with a life expectancy greater than 20 years. SAVR is also the preferred choice for patients with anatomic challenges, such as a bicuspid aortic stenosis (AS). However, they recommend using transcatheter aortic valve replacement (TAVR) primarily for patients who are over the age of 80 or who have life expectancies less than 10 years.
The ESC/EACTS 2025 guidelines suggest moving toward a greater use of TAVR for low surgical risk patients aged 75 or older and to allow for shared decision-making with patients aged 65–75. Although TAVR is possible in specific situations with patients with bicuspid aortic valves, there is no formal endorsement for its use in these cases.(47)
Both organizations emphasize the importance of incorporating multidisciplinary heart teams when performing risk assessments of patients. Multidisciplinary heart teams utilize the STS PROM scores, patient frailty, and patient preference as tools to evaluate a patient. In order to reduce the number of futile surgical interventions, all patients who are referred should be seen by an independent cardiologist and surgeon prior to making a decision regarding the type of surgical intervention that will be performed (i.e., TAVR or SAVR), should have imaging (CT calcium scoring) performed (if possible), and should undergo a holistic risk profile.
In addition, the framework established by the CMS mandates encourages the establishment of a formal heart team infrastructure for accreditation purposes. As a result, all patients who are treated with either TAVR or SAVR will be required to be entered into the designated data registry to ensure outcome tracking. It is through this collaborative approach that equity across institutions is achieved. An example of this would be the implementation of minimalist protocols that allow 80% of patients who are not at risk to be discharged within 24 hours. (48)
Measurement Niches
Long-term (>10 year) data for bicuspid TAVR are limited; however, registry data suggest that long term mortality rates from new-generation valves will be similar to tricuspid AS for the next 5-10 years although there is an increased incidence of strokes and unknown rates of SVD. New generation valves employ improved technology to address the problems of PPM/PVL; however, as the indications for TAVR increase, the feasibility of redo-TAVR and the need for explant will also increase. Data regarding the durability of TAVR in younger patients are largely unavailable as they are based upon extrapolation from the results of low-risk clinical trials such as PARTNER-3 at 7 year follow up.(49)
Clinical Applications
Data from PARTNER-3/Evolut LR suggest that TAVR will account for more than 70% of all low-risk AS patients in the United States. Clinical practice can utilize this knowledge to accurately counsel patients regarding their TAVR procedure. TAVR providers should include discussion of the following: potential PPM complications (e.g., HF and mortality), expectations of lifetime reinterventions (especially potential valve-in-valve interventions) and an assessment of the patient's frailty/clinical anatomy. Additional considerations for young patients with VHD and a bicuspid aortic valve may include educating the young patient about potential durability advantages associated with SAVR compared with the procedural safety of TAVR methodologies.
The future of ongoing prospective randomised trials such as NOTION-2 (which has 3 years of prospective data and has established non-inferiority to TAVR at ≤75 years old. A sub-analysis of the bicuspid subgroup is expected in the coming months) along with Evolut low risk bicuspid randomised studies, will allow for a more comprehensive understanding of the outcomes in a younger patient population. These studies are seeking to assess the ability of these types of next generation valves to maintain the level of safety for patients undergoing repeat procedures, and will include imaging to assist with valve selection prior to implantation. As more information becomes available from these prospective randomised trials, it is expected that the guidelines established prior to 2025 will evolve based on the outcomes of patients who survive ≥10 years and experience further procedures who will ultimately benefit from strategies established for their lifetime.(50)
Conclusion
The introduction of TAVR to the treatment of patients with severe symptomatic aortic stenosis at low surgical risk resulted in significant improvements in 30-day mortality rates, rehospitalization rates and recovery time compared to SAVR, but with the drawbacks of higher PPM/PVL rates and ongoing uncertainty surrounding the long-term durability of valves implanted via TAVR (especially in younger patients and those with bicuspid valves).(22,28,50) This unique combination of benefits and drawbacks, as well as the growing concern regarding the long-term durability of transcatheter valves, has led to the establishment of heart team based care for the ongoing lifetime management of patients with aortic stenosis as outlined in the current ACC/AHA and 2025 ESC/EACTS guidelines with preferences for SAVR in younger patients with a longer life expectancy and/or more complicated anatomy (ie. bicuspid aortic valves) and preference for extending TAVR use among older, lower-risk patients. (47,48) With the continued accumulation of data from long-term follow-up of low-risk TAVR clinical trials and studies specifically addressing younger patients and patients with bicuspid aortic valves, the aortic stenosis field will shift away from a simple, binary “TAVR or SAVR” decision-making process to a more individualized approach that includes thorough consideration of valve durability and reintervention plans, and patient preferences over the entire lifespan of the patient. (35,36,40,50)
References
-
Osnabrugge RLJ, Mylotte D, Head SJ, Van Mieghem NM, Nkomo VT, Lereun CM, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol [Internet]. 2013 Sep 10 [cited 2025 Dec 31];62(11):1002–12. Available from: https://pubmed.ncbi.nlm.nih.gov/23727214/
View at Publisher | View at Google Scholar -
Taleb Bendiab T, Brusset A, Estagnasié P, Squara P, Nguyen LS. Performance of EuroSCORE II and Society of Thoracic Surgeons risk scores in elderly patients undergoing aortic valve replacement surgery. Arch Cardiovasc Dis [Internet]. 2021 Jun 1 [cited 2025 Dec 31];114(6–7):474–81. Available from: https://pubmed.ncbi.nlm.nih.gov/33558164/
View at Publisher | View at Google Scholar -
The past, present and future of aortic stenosis treatment. British Journal of Cardiology. 2023;
View at Publisher | View at Google Scholar -
Bocchino PP, Angelini F, Alushi B, Conrotto F, Cioffi GM, Tersalvi G, et al. Transcatheter Aortic Valve Replacement in Young Low-Risk Patients With Severe Aortic Stenosis: A Review. Front Cardiovasc Med [Internet]. 2020 Dec 14 [cited 2025 Dec 31];7:608158. Available from: www.frontiersin.org
View at Publisher | View at Google Scholar -
Tödt J, Koul S, Yndigegn T, Angerås O, Bjursten H, Nozohoor S, et al. Percutaneous and surgical management of aortic stenosis in the SWEDEHEART registry (2013–2023): a nationwide observational study. The Lancet Regional Health - Europe [Internet]. 2026 Jan 1 [cited 2025 Dec 31];60:101520. Available from: https://www.thelancet.com/action/showFullText?pii=S2666776225003126
View at Publisher | View at Google Scholar -
Hamana T, Sekimoto T, Finn A V., Virmani R. Age Differences in Aortic Stenosis. Rev Cardiovasc Med [Internet]. 2025 Apr 1 [cited 2025 Dec 31];26(4):28185. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC12059746/
View at Publisher | View at Google Scholar -
Kausar H, Vishnoi A, Tripathi A. Evaluation of Long-Term Outcomes in Patients Undergoing Transcatheter Aortic Valve Replacement (TAVR) in Low-Risk Populations. European Journal of Cardiovascular Medicine [Internet]. 2025 Mar 28 [cited 2025 Dec 31];15:921–6. Available from: https://healthcare-bulletin.co.uk/article/evaluation-of-long-term-outcomes-in-patients-undergoing-transcatheter-aortic-valve-replacement-tavr-in-low-risk-populations-3375/
View at Publisher | View at Google Scholar -
Fu J, Popal MS, Li Y, Li G, Qi Y, Fang F, et al. Transcatheter versus surgical aortic valve replacement in low and intermediate risk patients with severe aortic stenosis: systematic review and meta-analysis of randomized controlled trials and propensity score matching observational studies. J Thorac Dis [Internet]. 2019 [cited 2025 Dec 31];11(5):1945–62. Available from: https://pubmed.ncbi.nlm.nih.gov/31285888/
View at Publisher | View at Google Scholar -
Fu J, Popal MS, Li Y, Li G, Qi Y, Fang F, et al. Transcatheter versus surgical aortic valve replacement in low and intermediate risk patients with severe aortic stenosis: systematic review and meta-analysis of randomized controlled trials and propensity score matching observational studies. J Thorac Dis [Internet]. 2019 [cited 2025 Dec 31];11(5):1945–62. Available from: https://pubmed.ncbi.nlm.nih.gov/31285888/
View at Publisher | View at Google Scholar -
Shimoda T, Miyamoto Y, Shimamura J, Ueyama H, Yokoyama Y, Sá MP, et al. Transcatheter versus surgical aortic valve replacement in low- to intermediate-risk patients: a meta-analysis of reconstructed time-to-event data. Ann Cardiothorac Surg [Internet]. 2025 Mar 31 [cited 2025 Dec 31];14(2):734–84. Available from: https://www.annalscts.com/article/view/17152/html
View at Publisher | View at Google Scholar -
Head SJ, Gahl B, Çelik M, Head SJ, Vanoverschelde JL, Pibarot P, et al. Natural History of Asymptomatic Severe Aortic Stenosis and the Association of Early Intervention With Outcomes: A Systematic Review and Meta-analysis. JAMA Cardiol [Internet]. 2020 Oct 1 [cited 2025 Dec 31];5(10):1. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC7344834/
View at Publisher | View at Google Scholar -
Lawrence JG, Carapetis JR, Griffiths K, Edwards K, Condon JR. Acute rheumatic fever and rheumatic heart disease: Incidence and progression in the Northern Territory of Australia, 1997 to 2010. Circulation. 2013 Jul 30;128(5):492–501.
View at Publisher | View at Google Scholar -
Nathaniel S, Saligram S, Innasimuthu AL. Aortic stenosis: An update. World J Cardiol [Internet]. 2010 [cited 2025 Dec 31];2(6):135. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC2999052/
View at Publisher | View at Google Scholar -
Pujari SH, Agasthi P. Aortic Stenosis. Congenital Cardiac Anesthesia: A Case-Based Approach [Internet]. 2023 Apr 16 [cited 2025 Dec 31];90–6. Available from: https://www.ncbi.nlm.nih.gov/books/NBK557628/
View at Publisher | View at Google Scholar -
Bevan GH, Zidar DA, Josephson RA, Al-Kindi SG. Mortality Due to Aortic Stenosis in the United States, 2008-2017. JAMA [Internet]. 2019 Jun 11 [cited 2025 Dec 31];321(22):2236. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC6563552/
View at Publisher | View at Google Scholar -
Patel K V., Omar W, Gonzalez PE, Jessen ME, Huffman L, Kumbhani DJ, et al. Expansion of TAVR into Low-Risk Patients and Who to Consider for SAVR. Cardiol Ther [Internet]. 2020 Dec 1 [cited 2025 Dec 31];9(2):377. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC7584721/
View at Publisher | View at Google Scholar -
Virtanen MPO, Eskola M, Jalava MP, Husso A, Laakso T, Niemelä M, et al. Comparison of Outcomes After Transcatheter Aortic Valve Replacement vs Surgical Aortic Valve Replacement Among Patients With Aortic Stenosis at Low Operative Risk. JAMA Netw Open [Internet]. 2019 Jun 5 [cited 2025 Dec 31];2(6):e195742. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC6575142/
View at Publisher | View at Google Scholar -
Kolte D, Vlahakes GJ, Palacios IF, Sakhuja R, Passeri JJ, Inglessis I, et al. Transcatheter Versus Surgical Aortic Valve Replacement in Low-Risk Patients. J Am Coll Cardiol [Internet]. 2019 Sep 24 [cited 2025 Dec 31];74(12):1532–40. Available from: https://pubmed.ncbi.nlm.nih.gov/31537261/
View at Publisher | View at Google Scholar -
https://fyra.io. TAVR in Patients With Aortic Stenosis and Low Surgical Risk - Cardiac Interventions Today. [cited 2025 Dec 31]; Available from: https://citoday.com/articles/2019-mar-apr/tavr-in-patients-with-aortic-stenosis-and-low-surgical-risk
View at Publisher | View at Google Scholar -
Virtanen MPO, Eskola M, Jalava MP, Husso A, Laakso T, Niemelä M, et al. Comparison of Outcomes After Transcatheter Aortic Valve Replacement vs Surgical Aortic Valve Replacement Among Patients With Aortic Stenosis at Low Operative Risk. JAMA Netw Open [Internet]. 2019 Jun 5 [cited 2025 Dec 31];2(6):e195742–e195742. Available from: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2735762
View at Publisher | View at Google Scholar -
Jabri A, Alameh A, Giustino G, Gonzalez PE, O’Neill B, Bagur R, et al. Transcatheter Aortic Valve Replacement is Ready for Most Low-risk Patients: A Systematic Review of the Literature. Card Fail Rev [Internet]. 2024 [cited 2025 Dec 31];10:e11. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC11462515/
View at Publisher | View at Google Scholar -
Popma JJ, Deeb GM, Yakubov SJ, Mumtaz M, Gada H, O’Hair D, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Valve in Low-Risk Patients. N Engl J Med [Internet]. 2019 May 2 [cited 2025 Dec 31];380(18):1706–15. Available from: https://pubmed.ncbi.nlm.nih.gov/30883053/
View at Publisher | View at Google Scholar -
Reddy RK, Howard JP, Mack MJ, Reardon MJ, Jørgensen TH, Hørsted Thyregod HG, et al. Transcatheter vs Surgical Aortic Valve Replacement in Lower-Risk Patients: An Updated Meta-Analysis of Randomized Controlled Trials. J Am Coll Cardiol [Internet]. 2025 Mar 11 [cited 2025 Dec 31];85(9):926–40. Available from: https://pubmed.ncbi.nlm.nih.gov/40044297/
View at Publisher | View at Google Scholar -
Hørsted Thyregod HG, Jørgensen TH, Ihlemann N, Steinbrüchel DA, Nissen H, Kjeldsen BJ, et al. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur Heart J [Internet]. 2024 Apr 1 [cited 2025 Dec 31];45(13):1116–24. Available from: https://dx.doi.org/10.1093/eurheartj/ehae043
View at Publisher | View at Google Scholar -
Ahmed M, Ahsan A, Shafiq A, Nadeem ZA, Arif F, Zulfiqar E, et al. Meta-analysis of longitudinal comparison of transcatheter versus surgical aortic valve replacement in patients at low to intermediate surgical risk. Int J Surg [Internet]. 2024 Dec 1 [cited 2025 Dec 31];110(12):8097. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC11634167/
View at Publisher | View at Google Scholar -
Kazemian S, Fallahtafti P, Sharifi M, Mohammadi NSH, Soleimani H, Moghadam AS, et al. Trends in Transcatheter Versus Surgical Aortic Valve Replacement Outcomes in Patients With Low‐Surgical Risk: A Systematic Review and Meta‐Analysis of Randomized Trials. J Am Heart Assoc [Internet]. 2024 Nov 5 [cited 2025 Dec 31];13(21):36179. Available from: /doi/pdf/10.1161/JAHA.124.036179?download=true
View at Publisher | View at Google Scholar -
Krishna MM, Joseph M, Ezenna C, Pereira V, Rossi R, Akman Z, et al. TAVR vs. SAVR for severe aortic stenosis in the low and intermediate surgical risk population: An updated meta-analysis, meta-regression, and trial sequential analysis. Cardiovasc Revasc Med [Internet]. 2025 Oct 1 [cited 2025 Dec 31];79:78–89. Available from: https://pubmed.ncbi.nlm.nih.gov/40425422/
View at Publisher | View at Google Scholar -
Mack MJ, Leon MB, Thourani VH, Makkar R, Kodali SK, Russo M, et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N Engl J Med [Internet]. 2019 May 2 [cited 2025 Dec 31];380(18):1695–705. Available from: https://pubmed.ncbi.nlm.nih.gov/30883058/
View at Publisher | View at Google Scholar -
Kheiri B, Osman M, Abubakar H, Subahi A, Chahine A, Ahmed S, et al. Transcatheter versus surgical aortic valve replacement in low-risk surgical patients: A meta-analysis of randomized clinical trials. Cardiovasc Revasc Med [Internet]. 2019 Oct 1 [cited 2025 Dec 31];20(10):838–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/30638890
View at Publisher | View at Google Scholar -
Altaii H, Morcos R, Riad FS, Abdulameer H, Khalili H, Maini B, et al. Incidence of Early Atrial Fibrillation After Transcatheter versus Surgical Aortic Valve Replacement: A Meta-Analysis of Randomized Controlled Trials. J Atr Fibrillation [Internet]. 2020 Dec 1 [cited 2025 Dec 31];13(4):2411. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8691290/
View at Publisher | View at Google Scholar -
Altaii H, Morcos R, Riad FS, Abdulameer H, Khalili H, Maini B, et al. Incidence of Early Atrial Fibrillation After Transcatheter versus Surgical Aortic Valve Replacement: A Meta-Analysis of Randomized Controlled Trials. J Atr Fibrillation [Internet]. 2020 Dec 1 [cited 2025 Dec 31];13(4):2411. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8691290/
View at Publisher | View at Google Scholar -
Jabri A, Alameh A, Giustino G, Gonzalez PE, O’Neill B, Bagur R, et al. Transcatheter Aortic Valve Replacement is Ready for Most Low-risk Patients: A Systematic Review of the Literature. Card Fail Rev [Internet]. 2024 [cited 2025 Dec 31];10:e11. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC11462515/
View at Publisher | View at Google Scholar -
Mach M, Okutucu S, Kerbel T, Arjomand A, Fatihoglu SG, Werner P, et al. Vascular complications in tavr: Incidence, clinical impact, and management. J Clin Med [Internet]. 2021 Nov 1 [cited 2025 Dec 31];10(21):5046. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8584339/
View at Publisher | View at Google Scholar -
Forrest JK, Deeb GM, Yakubov SJ, Gada H, Mumtaz MA, Ramlawi B, et al. 3-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J Am Coll Cardiol [Internet]. 2023 May 2 [cited 2025 Dec 31];81(17):1663–74. Available from: https://pubmed.ncbi.nlm.nih.gov/36882136/
View at Publisher | View at Google Scholar -
Forrest JK, Yakubov SJ, Deeb GM, Gada H, Mumtaz MA, Ramlawi B, et al. 5-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J Am Coll Cardiol [Internet]. 2025 Apr 22 [cited 2025 Dec 31];85(15):1523–32. Available from: http%3a%2f%2fwww.acc.org%2fLatest-in-Cardiology%2fJournal-Scans%2f2025%2f03%2f24%2f16%2f30%2fsun-10am-evolut-acc-2025
View at Publisher | View at Google Scholar -
TCT 2025 | PARTNER 3 Trial 7-Year Outcomes: TAVR vs SAVR in Low-Risk Patients [Internet]. [cited 2025 Dec 31]. Available from: https://solaci.org/en/2025/10/29/tct-2025-partner-3-trial-7-year-outcomes-tavr-vs-savr-in-low-risk-patients/
View at Publisher | View at Google Scholar -
Ferrarotto L, Immè S, Tamburino C, Tamburino C. Ten years of transcatheter aortic valve implantation in the NOTION study: the good and the bad. Eur Heart J Suppl [Internet]. 2025 Mar 1 [cited 2025 Dec 31];27(Suppl 3):iii153. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC12001784/
View at Publisher | View at Google Scholar -
Baron SJ, Arnold S V., Wang K, Magnuson EA, Chinnakondepali K, Makkar R, et al. Health Status Benefits of Transcatheter vs Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk: Results From the PARTNER 2 Randomized Clinical Trial. JAMA Cardiol [Internet]. 2017 Aug 1 [cited 2025 Dec 31];2(8):837–45. Available from: https://pubmed.ncbi.nlm.nih.gov/28658491/
View at Publisher | View at Google Scholar -
Modine T, Tchétché D, Van Mieghem NM, Michael Deeb G, Chetcuti SJ, Yakubov SJ, et al. Three-Year (<75 Years)Outcomes Low-Surgical-Risk FollowingSevere TAVR inAortic Younger Stenosis Patients. Circ Cardiovasc Interv [Internet]. 2024 Nov 1 [cited 2025 Dec 31];17(11):e014018. Available from: /doi/pdf/10.1161/CIRCINTERVENTIONS.124.014018?download=true
View at Publisher | View at Google Scholar -
Zahr F, Ramlawi B, Reardon MJ, Deeb GM, Yakubov SJ, Song HK, et al. 3-Year Outcomes From the Evolut Low Risk TAVR Bicuspid Study. JACC Cardiovasc Interv [Internet]. 2024 Jul 22 [cited 2025 Dec 31];17(14):1667–75. Available from: https://pubmed.ncbi.nlm.nih.gov/39048253/
View at Publisher | View at Google Scholar -
Coylewright M, Grubb KJ, Arnold S V., Batchelor W, Dhoble A, Horne A, et al. Outcomes of Balloon-Expandable Transcatheter Aortic Valve Replacement in Younger Patients in the Low-Risk Era. JAMA Cardiol [Internet]. 2025 Feb 1 [cited 2025 Dec 31];10(2):127–35. Available from: https://jamanetwork.com/journals/jamacardiology/fullarticle/2825505
View at Publisher | View at Google Scholar -
Sharaf OM, Beaver TM. Aortic valve 2024: Which valve for which patient? [cited 2025 Dec 31]; Available from: https://doi.org/10.1016/j.jtcvs.2024.06.023
View at Publisher | View at Google Scholar -
Rao K, Baer A, Bapat VN, Piazza N, Hansen P, Prendergast B, et al. Lifetime management considerations to optimise transcatheter aortic valve implantation: a practical guide. EuroIntervention [Internet]. 2024 Dec 16 [cited 2025 Dec 31];20(24):e1493–504. Available from: https://pubmed.ncbi.nlm.nih.gov/39676551/
View at Publisher | View at Google Scholar -
Leon MB, Mack MJ, Pibarot P, Hahn RT, Thourani VH, Kodali SH, et al. Transcatheter or Surgical Aortic-Valve Replacement in Low-Risk Patients at 7 Years. New England Journal of Medicine. 2025 Oct 27;
View at Publisher | View at Google Scholar -
Mehta CK, Liu TX, Baldridge AS, Kruse J, Puthumana J, Bonow RO, et al. Long-term durability of bioprosthetic aortic valve replacement in young patients with bicuspid aortic stenosis. JTCVS Structural and Endovascular [Internet]. 2024 Sep 1 [cited 2025 Dec 31];1–2:100004. Available from: https://www.sciencedirect.com/science/article/pii/S2950605024000044
View at Publisher | View at Google Scholar -
Forrest JK, Yakubov SJ, Deeb GM, Gada H, Mumtaz MA, Ramlawi B, et al. 5-Year Outcomes After Transcatheter or Surgical Aortic Valve Replacement in Low-Risk Patients With Aortic Stenosis. J Am Coll Cardiol. 2025 Apr 22;85(15):1523–32.
View at Publisher | View at Google Scholar -
Praz F, Borger MA, Lanz J, Marin-Cuartas M, Abreu A, Adamo M, et al. 2025 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2025 Nov 21;46(44):4635–736.
View at Publisher | View at Google Scholar -
Hussain K, Huerter M. ACC/AHA Guidelines for Valve Disease. StatPearls [Internet]. 2024 Jun 8 [cited 2025 Dec 31]; Available from: https://www.ncbi.nlm.nih.gov/books/NBK606116/
View at Publisher | View at Google Scholar -
Walczewski M, Gasecka A, Huczek Z, Rymuza B, Kochman J. Ten-year experience with transcatheter aortic valve implantation in bicuspid aortic valve: lessons learned and future perspectives. Postepy Kardiol Interwencyjnej [Internet]. 2021 [cited 2025 Dec 31];17(3):251. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC8596720/
View at Publisher | View at Google Scholar -
Ng VG, Kodali SK, Leon MB. TAVR under 70: is age just a number? EuroIntervention [Internet]. 2022 Mar 1 [cited 2025 Dec 31];17(16):1281. Available from: https://pmc.ncbi.nlm.nih.gov/articles/PMC9743249/
View at Publisher | View at Google Scholar






