Research Article | DOI: https://doi.org/CCSRR-RA-25-023.
Role of High-Density Lipoprotein in Cardiovascular Risk: A Review
Abstract
High-density lipoprotein (HDL), known as "good" cholesterol, plays a vital role in heart health by helping remove excess cholesterol from tissues and transporting it to the liver for disposal. This process, called reverse cholesterol transport (RCT), not only lowers the risk of artery blockages (atherosclerosis) but also has anti-inflammatory and antioxidant effects, benefiting blood vessel function. This review explores HDL's structure, how it works in the body, and its roles. HDL contains proteins like ApoA-I that interact with enzymes and cell receptors to remove cholesterol. Genetic differences in proteins like APOA1, CETP, and ABCA1 can affect how HDL functions, influencing cardiovascular risk. Mechanistically, HDL enhances endothelial function by promoting nitric oxide synthesis, inhibits inflammation by reducing cytokine production, and stabilizes atherosclerotic plaques by preventing LDL oxidation. Furthermore, HDL's role in plaque stabilization and its immunomodulatory effects underscore its therapeutic potential in cardiovascular disease management. Clinical implications highlight the importance of assessing HDL functionality beyond absolute levels in predicting cardiovascular risk. Current therapeutic strategies focus on optimizing lipid profiles through lifestyle modifications and pharmacological interventions targeting HDL metabolism. Future directions include HDL mimetics, genetic studies, and personalized medicine approaches to enhance HDL functionality and mitigate cardiovascular risk effectively. In conclusion, while HDL's role in cardiovascular protection is complex and multifaceted, understanding its structural components, metabolic pathways, and genetic determinants is crucial for developing targeted therapies and advancing personalized medicine in cardiovascular health.
Abbreviation:
ABCA1 - ATP-binding cassette transporter A1
ABCG1 - ATP-binding cassette transporter G1
APOA1 - apolipoprotein A-I
APOC3 - apolipoprotein C-III
APOE - apolipoprotein E
CETP - Cholesteryl ester transfer protein
CVD - cardiovascular disease
eNOs – endothelial nitric oxide synthase
GWAS - Genome-wide association studies
HDL - High-density lipoprotein
ICAM – 1 – Intercellular adhesion molecule 1
IL-1 - Interleukin-1
IL-6 - Interleukin-6
LCAT - Lecithin-cholesterol acyltransferase
LDL - Low-density lipoprotein
LIPC- Hepatic Lipase gene
LRP - LDL receptor-related protein
LXR - liver X receptor agonists
NO - nitric oxide
PLTP - phospholipid transfer protein
RCT - Reverse cholesterol transport
SR-BI - Scavenger receptor class B type I
TICE - trans-intestinal excretion
TNF-alpha - Tumour necrosis factor-alpha
VCAM – 1 – Vascular cell adhesion molecule 1
Introduction
Lipoproteins are vital for heart health by transporting cholesterol and lipids. LDL, or "bad" cholesterol, can cause plaque build-up in arteries, raising the risk of atherosclerosis. Conversely, HDL, or "good" cholesterol, removes excess cholesterol, reducing cardiovascular disease risk. Maintaining a balance between LDL and HDL is critical for heart health. Although the liver and intestine are the main sites of formation for high-density lipoprotein particles, peripheral tissues such as the small intestine, adipose tissue, and macrophages can also produce these particles. The primary structural proteins of high-density lipoproteins are apolipoproteins, particularly ApoA-I, which are crucial for stabilizing the particle and facilitating its interactions with enzymes and cell receptors. HDL return extra cholesterol from peripheral tissues—such as artery walls—to the liver where it is eliminated as bile. This procedure lowers the risk of all major cardiovascular illnesses by preventing the accumulation of cholesterol in blood arteries. In addition to their cardioprotective qualities, HDL also have anti-inflammatory, antioxidant, and antithrombotic actions. It improves endothelial function and reduces LDL oxidation, which encourages atherosclerotic plaque development.[1]
HDL is "good" cholesterol, crucial in lipid metabolism, aids cardiovascular health. The role of HDL includes:
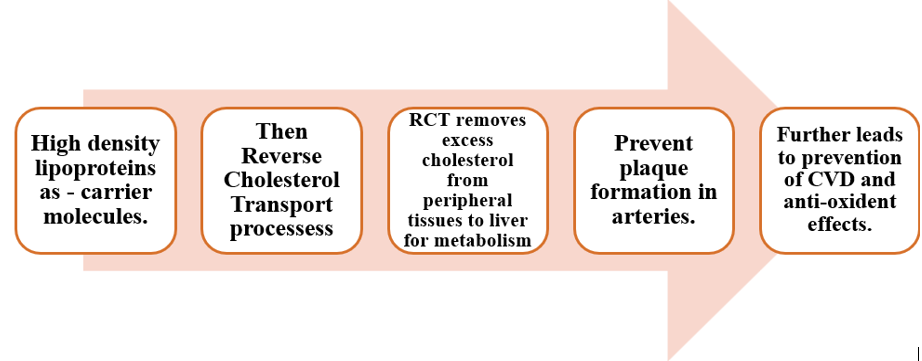
1. RCT: HDL picks up excess cholesterol for excretion in bile and helps to prevent the accumulation of cholesterol in blood vessels, reducing the risk of atherosclerosis and cardiovascular disease.[2]
2. Anti-inflammatory and antioxidant properties: Antioxidant qualities of high-density lipoproteins are extremely advantageous. These help to counteract the harmful free radicals that trigger LDL cholesterol to oxidize. The likelihood of these oxidized LDLs causing atherosclerosis is higher. Furthermore, HDLs have an anti-inflammatory property that aids in lowering blood vessel inflammation. These two procedures are essential for preventing atherosclerosis.
3. Vasodilatation and Endothelial function: HDL supports endothelial cell health by increase nitric oxide synthesis, which is a potent vasodilator. Vasodilatation, triggered on by nitric oxide, enhances circulation and aids in preserving normal blood pressure. Maintains cardiovascular health by supporting vascular function and shield endothelium cells against harm and malfunction. [3]
Biology of Hdl:
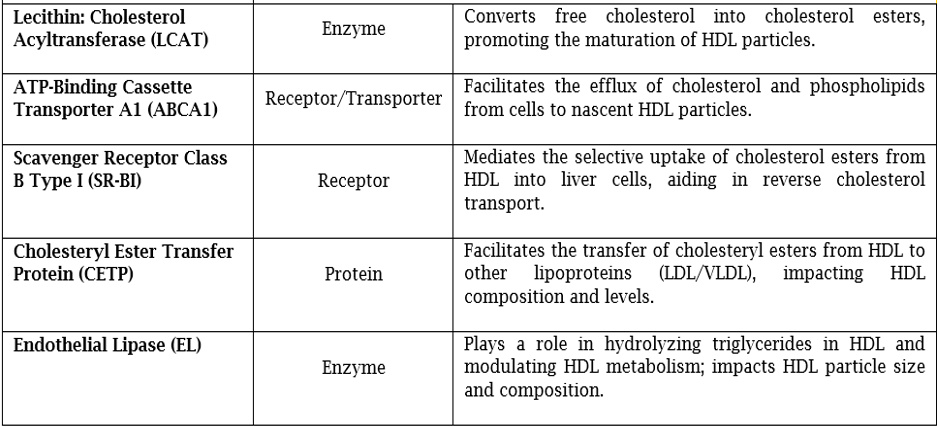
HDL, the smallest lipoprotein, surrounds cells, comprising proteins and lipids. Its varied sub-species reveal diverse physiological roles. Lipids arrange in micelle-like structures, HDL's lipid composition is crucial for its physiological functions, primarily involving cholesterol transport. It consists of a core of triglycerides and cholesteryl esters, surrounded by a monolayer of phospholipids, with free cholesterol essential for particle fluidity and cholesterol efflux and uptake [5,6]. HDL proteins and lipids vary greatly in concentration, with each particle carrying cholesterol, phosphatidylcholine, apoA-I, and apolipoprotein A-II (ApoA-II) [7]. HDL's primary apolipoprotein, apoA-I, maintains its structure and aids in removing excess cellular cholesterol through ABCA1. HDL typically forms large spherical structures with at least three apoA-I molecules or discoidal shapes with two apoA-I copies. Other proteins on HDL, like LCAT, CETP, and paraoxonase-1, have crucial but low-abundance roles. Ancillary proteins, over 200 in number, include haptoglobin and alpha-1 antitrypsin, aiding in unique biological functions like trypanosome lysis and inflammation suppression. HDL's structural role extends beyond phospholipids to prevent lipoprotein-X (Lp-X) formation, crucial for averting kidney disease. Enzymes like LCAT process phospholipids into bioactive molecules and crucial for the esterification of free cholesterol, which enables HDL to transport cholesterol more efficiently. Triglyceride transfer by CETP aids post-prandial lipid delivery to tissues. Plasma Paraoxonase 1 (PON1) is an enzyme that plays a crucial role in HDL function, providing antioxidant properties and protecting it from oxidation, which can lead to atherogenesis [8]. Sphingosine-1 phosphate on HDL has biological signalling roles. HDL also interacts with miRNAs, though implications remain unclear. Recent proteomic analyses and metabolic turnover studies indicate that HDL sub-classes maintain a stable core-protein composition throughout their lifecycle. These sub-classes often contain specialized proteins with related functions like haemostasis or protease inhibition. HDL's lipid composition is dynamic, with ABCA1, LCAT, endothelial lipase, and hepatic lipase playing key roles in lipid metabolism. CETP and PLTP facilitate lipid exchange between HDL and other lipoproteins. HDL also transports bioactive molecules like S1P, contributing to its multifaceted functions in cardiovascular health [9,10].
Physiology of Hdl metabolism:
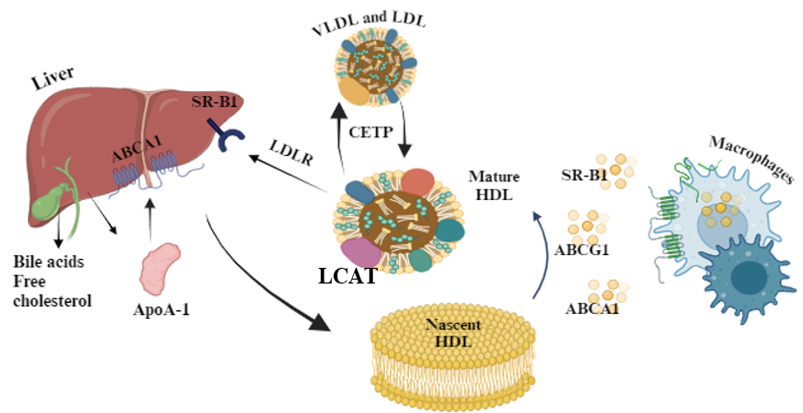
1. Synthesis of HDL Cholesterol:
• HDL cholesterol- ApoA-I is primarily synthesized in the liver and intestine.
• ApoA-I interacts with the ABCA1 cholesterol-phospholipid transporter, which is expressed by hepatocytes and enterocytes to acquire lipids, thereby producing nascent HDL particles.
• These nascent HDL particles then acquire additional lipids, including cholesterol and phospholipids, from peripheral tissues through a process known as reverse cholesterol transport [11].
2. Transport of HDL Cholesterol:
• HDL particles circulate in the bloodstream and interact with various tissues and organs to facilitate the removal of excess cholesterol.
• HDL interacts with ABCA1 transporter on peripheral cells, such as macrophages, to accept cholesterol and phospholipids, forming pre-beta HDL particles.
• Pre-beta HDL is then converted to alpha HDL through the action of LCAT, which esterifies free cholesterol, making it more hydrophobic and allowing it to be incorporated into the HDL core [12].
3. Role of Enzymes, Receptors, and Transport Proteins:
• LCAT: Converts pre-beta HDL to alpha HDL by esterifying free cholesterol [12].
• ABCA1 Transporter: Mediates reverse cholesterol transport and prevents detrimental lipid deposition [13].
• SR-B1: Located on the surface of hepatocytes and steroidogenic cells. Contributes in cholesterol metabolism and steroid hormone synthesis [14].
• CETP: Influence overall lipid metabolism and cardiovascular risk [15].
4. Catabolism of HDL Cholesterol:
• Mature HDL particles undergo catabolism primarily in the liver, where they are taken up via receptor-mediated endocytosis.
• Hepatocytes express receptors such as SR-B1 and LDL receptor-related protein (LRP) that facilitate the uptake of HDL cholesterol esters, which are subsequently hydrolyzed by hepatic lipase, releasing free cholesterol for excretion into bile or conversion to bile acids [16].
MECHANISMS OF HDL – MEDIATED CARDIOVASCULAR PROTECTION
1. Reverse Cholesterol Transport:
Macrophages and foam cells in artery walls are crucial for extracting cholesterol via HDL particles. This process is known as RCT, aids in eliminating excess cholesterol from cells to the liver for excretion [17]. HDL's ability to efflux cholesterol may better indicate cardiovascular risk than HDL-C levels; pivotal in atheroprotection [18]. HDL must traverse the endothelium to access arterial intimal cells for cholesterol outflow. Endothelial cells engage HDL via SR-BI and ABCG1, facilitating its translocation from apical to basolateral compartments. Similarly, lipid-free apoA-I transcytosis, aided by ABCA1, enables lipidation. HDL then interacts with cell receptors, initiating selective or non-specific cholesterol efflux [19]. ABCA1 and ABCG1 mediate active, unidirectional efflux, while SR-BI facilitates passive, bidirectional transfer through diffusion [20]. ABCA1, a critical multi-pass transporter, drives over 80% of cholesterol efflux from loaded cells. This process relies on lipid-free/lipid-poor apolipoproteins and small pre-beta HDL particles, vital for mature HDL formation [21]. ABCG1, along with ABCA1 and LCAT, contributes to the formation of large HDL2 and HDL3 particles through cholesterol efflux. While ABCG1 aids in HDL synthesis, its role in cholesterol efflux from macrophages appears quantitatively less significant than ABCA1 [22]. SR-BI enables bidirectional, ATP-independent cholesterol flux across mature HDL and plasma membranes, primarily in hepatocytes but also in macrophages, adipocytes, and other cells [23].
RCT is believed to play a significant role in preventing atherosclerosis [24]. Through the RCT pathway; HDL carries extra cholesterol from peripheral organs, smooth muscle cells, and foamy macrophages to the liver. HDL carries cholesterol to the liver, where it is partially eliminated as bile and partially retained as cholesteryl esters [18]. Cholesterol efflux into the intestinal system is significantly influenced by RCT and TICE [25].
2. Anti-inflammatory Properties: By modifying different inflammatory pathways linked to atherosclerosis and cardiovascular disorders, HDL has anti-inflammatory properties [26]. It lessens adhesion and the recruitment of inflammatory cells into the artery wall by inhibiting the production of adhesion molecules on endothelial cells, such as VCAM-1 and ICAM-1 [27]. By inhibiting the synthesis of pro-inflammatory cytokines like TNF-alpha, IL-1, and IL-6, HDL reduces inflammation in the artery wall [28]. Moreover, HDL prevents LDL cholesterol from being oxidized, which stops the production of oxidized LDL particles, which are very pro-inflammatory and lead to endothelial dysfunction and atherosclerosis [29].
3. Endothelial Function: HDL enhances endothelial function by increasing the production and bioavailability of endothelial NO, a potent vasodilator that regulates vascular tone, improves blood flow, and inhibits platelet aggregation [30]. HDL stimulates endothelial NO synthase (eNOs) activity, leading to increased NO production by endothelial cells. HDL also inhibits endothelial cell apoptosis, preserving endothelial integrity and reducing endothelial dysfunction risk. It promotes endothelial repair processes and angiogenesis, contributing to vascular remodeling and repairs. These actions help maintain vascular health, prevent atherosclerosis, and reduce cardiovascular disease risk [31].
4. Role of HDL in Plaque stabilization: HDL plays a crucial role in plaque stabilization primarily through its ability to promote RCT. In RCT, extra cholesterol is extracted from peripheral tissues—including those within atherosclerotic plaques—and sent to the liver for excretion [32]. HDL contributes to the stabilization of the composition of plaques by reducing lipid accumulation and promoting the outflow of cholesterol from macrophages within plaques. Inhibiting the growth of plaque and its susceptibility to rupture is a crucial step in the pathophysiology of acute cardiovascular events, including myocardial infarction and stroke [33]. Furthermore, by lowering oxidative stress and inflammation in the plaque microenvironment, HDL's anti-inflammatory and antioxidant characteristics help to stabilize the plaque. In general, HDL's role in stabilizing plaque lowers the risk of problems associated to plaque and preserves arterial integrity [34].
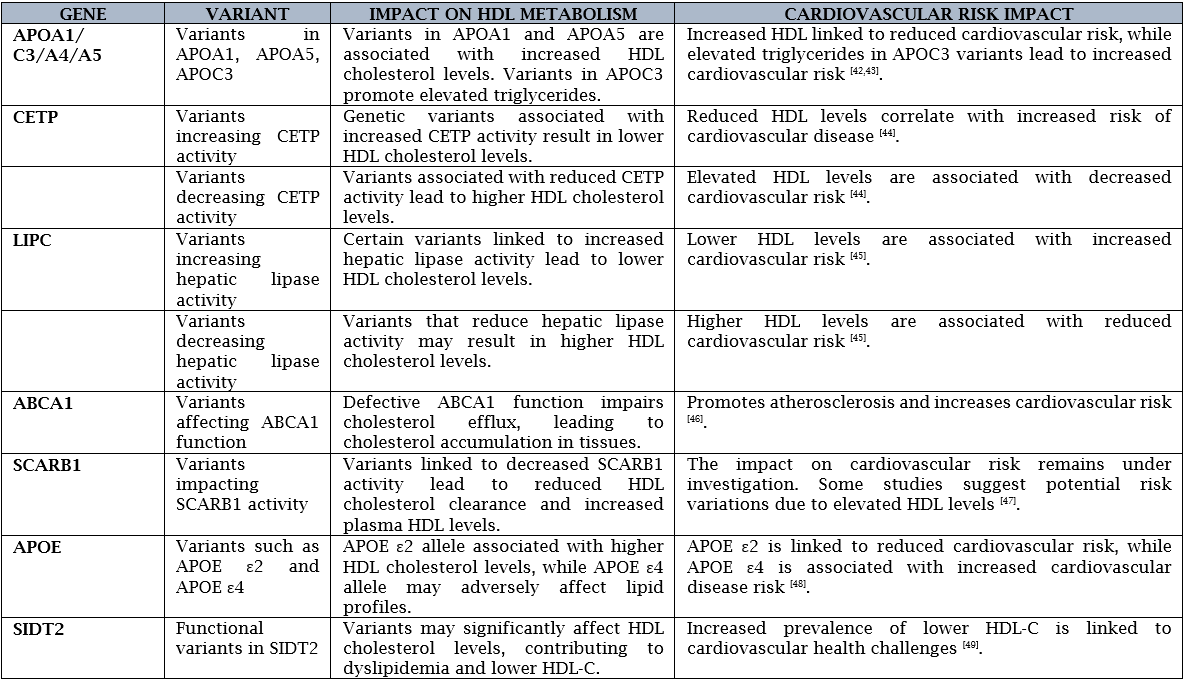
GENETICS AND HDL:
Various genetic variants associated with HDL metabolism and their impact on cardiovascular risk:
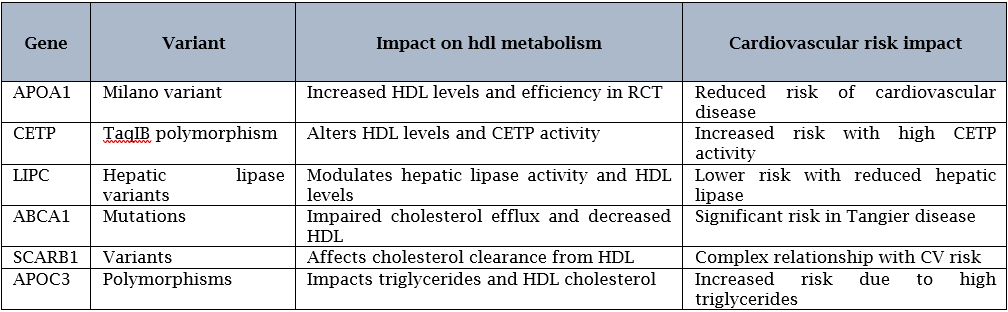
APOA1 variants: APOA1 the major protein component of HDL particles. Mutations in APOA1 can influence the production and function of HDL particles [35].
CETP variants: The CETP gene, which facilitates the transfer of cholesteryl esters from HDL to other lipoproteins. This dysregulation in HDL metabolism may contribute to an increased risk of cardiovascular disease, particularly in the presence of elevated levels of atherogenic lipoproteins [36].
LIPC variants: Hepatic lipase (LIPC) is an enzyme involved in the hydrolysis of triglycerides and phospholipids in lipoproteins, including HDL. Individuals carrying these variants may have a decreased risk of cardiovascular disease due to the protective effects of elevated HDL levels on lipid metabolism and atherosclerosis [37].
ABCA1 variants: ABCA1 gene which plays a crucial role in the process of RCT by facilitating the efflux of cholesterol from peripheral tissues to HDL particles [38].
SCARB1 variants: SCARB1 is involved in the selective uptake of cholesterol from HDL particles by the liver and other tissues [39].
APOC3 variants: APOC3, which plays a role in the regulation of triglyceride-rich lipoproteins [40].
ANGPTL3: Loss-of-function variations in the angiopoietin-like 3 gene (ANGPTL3) have been linked to elevated HDL cholesterol and decreased triglyceride and low-density lipoprotein (LDL) cholesterol levels. The significance of ANGPTL3 in lipid metabolism and its potential as a therapeutic target to reduce cardiovascular risks are highlighted by these genetic changes [41].
GWAS (Genome-wide association study) have identified several genetic loci associated with HDL-related genes, including the APOA1/C3/A5 gene cluster, CETP gene, LIPC gene, ABCA1 gene, SCARB1 gene, and APOE gene, and their relationship with cardiovascular outcomes. Here’s an overview of the findings:
Clinical Implications:
Short steps outlining the clinical relevance of HDL levels in risk prediction of cardiovascular disease: [50]
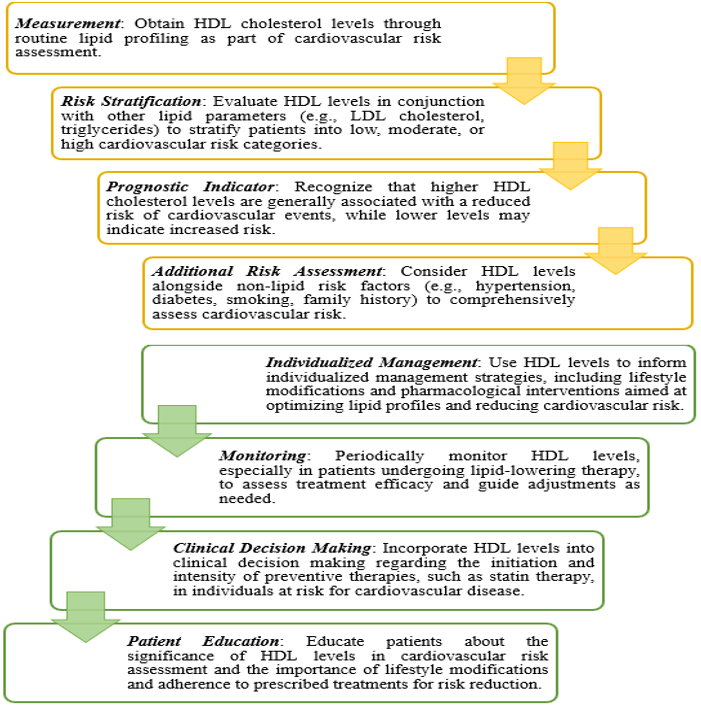
HDL levels are often included in lipid profiles for CVD risk assessment. Low HDL levels (<40>
The clinical relevance of HDL levels in the treatment of CVD lies in their role as a biomarker for risk assessment and guiding therapeutic strategies. Higher HDL cholesterol levels are generally considered cardio protective. However, interventions solely targeting HDL levels have shown limited efficacy in reducing cardiovascular events. Instead, a comprehensive approach that addresses overall lipid profile, lifestyle factors, and other modifiable risk factors is essential for effective CVD prevention and management [52].
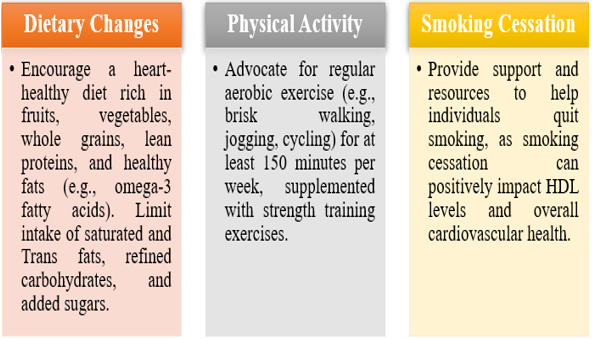
I. Optimizing Lipid Levels:
• Statins: Initiate statin therapy as first-line pharmacological treatment for individuals with elevated LDL cholesterol levels, regardless of HDL levels. Statins have been shown to reduce cardiovascular events and may modestly increase HDL cholesterol levels. Combination Therapy: Consider combination therapy with statins and other lipid-lowering agents (e.g., ezetimibe, PCSK9 inhibitors) for individuals at high cardiovascular risk or with persistent dyslipidaemia despite statin therapy. Fibrate Therapy: In selected individuals with hypertriglyceridemia and low HDL cholesterol levels, fibrates may be considered to improve lipid profile and reduce cardiovascular risk. Niacin: Niacin (nicotinic acid) can increase HDL levels but has fallen out of favour due to its adverse effects and lack of consistent cardiovascular benefits in clinical trials.
II. Management of Co morbidities:
• Hypertension Control: Ensure optimal blood pressure control through lifestyle modifications and pharmacological therapy (e.g., angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, diuretics). Diabetes Management: Implement strategies for glycaemic control through lifestyle modifications, antidiabetic medications (e.g., metformin, sulfonylureas, SGLT2 inhibitors, GLP-1 receptor agonists), and insulin therapy as needed.
III. Assessment of HDL Functionality:
• While routine clinical assays primarily measure HDL cholesterol levels, consider research-based or specialized tests to assess HDL functionality.
IV. Individualized Approach:
• Tailor treatment strategies based on individual patient characteristics, including age, gender, genetic predispositions, comorbidities, concomitant medications, and patient preferences. Regularly monitor lipid profiles, including HDL cholesterol levels, and adjust treatment regimens as needed to achieve optimal cardiovascular risk reduction. While directly targeting HDL levels as a treatment approach has limitations, optimizing HDL levels through lifestyle modifications, lipid-lowering therapy, management of comorbidities, and individualized treatment strategies can contribute to comprehensive cardiovascular risk reduction and management [53,54,55,56].
Future Directions:
i. HDL Mimetics and Modulators: Developing synthetic HDL particles to mimic the beneficial functions of natural HDL, such as reverse cholesterol transport and anti-inflammatory properties [57]. Investigating compounds that can selectively modulate HDL metabolism and function, potentially enhancing its atheroprotective effects [58].
ii. Genetic Approaches: Studying genetic variations associated with HDL metabolism to identify novel therapeutic targets and pathways for intervention [59].
iii. Microbiome Influence: Exploring the role of gut microbiota in HDL metabolism and considering strategies to manipulate the microbiome to improve HDL levels and function [60,61].
iv. Epigenetic Regulation: Understanding epigenetic mechanisms that regulate HDL metabolism and exploring targeted interventions to modify gene expression and enhance HDL function [62].
v. Nanotechnology: Utilizing nanotechnology to design targeted delivery systems for HDL-modulating agents, enhancing their efficacy and minimizing off-target effects. [figure3] [63,64]
vi. Immunomodulation: Investigating the immunomodulatory functions of HDL and exploring strategies to harness these properties for therapeutic purposes, particularly in inflammatory conditions associated with cardiovascular disease [64,65].
vii. Metabolic Syndrome: Studying the impact of metabolic syndrome on HDL metabolism and developing tailored therapeutic approaches to address dyslipidaemia and related metabolic abnormalities [66].
viii. Emerging Therapeutics: Assessing the efficacy and safety of novel HDL-targeted therapies, such as apoA-I mimetic peptides, CETP (cholesteryl ester transfer protein) inhibitors, and LXR (liver X receptor) agonists, in clinical trials. [figure3] [67,68]
ix. Precision Medicine: Advancing personalized approaches to HDL-targeted therapy by considering individual genetic, metabolic, and environmental factors to optimize treatment outcomes.
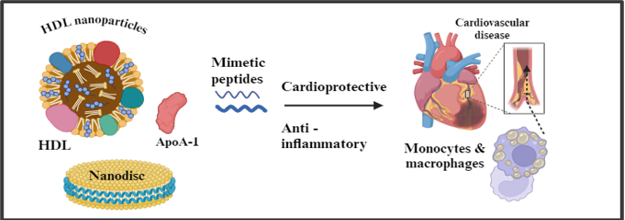
Recent developments in HDL therapeutics have steered away from simply boosting HDL cholesterol levels towards enhancing HDL function, specifically its ability to remove cholesterol—a function compromised in cardiovascular patients. Both pre-clinical and clinical investigations have substantiated the efficacy of HDL mimetic nanoparticles in bolstering cholesterol efflux, thereby stabilizing atherosclerotic plaques through anti-inflammatory pathways. Moreover, these nanoparticles exhibit immunomodulatory effects on immune cells like monocytes and macrophages, endowing them with multiple cardioprotective attributes that enhance vascular health. The ongoing phase III AEGIS-II trial assessing CSL112 offers promising insights, potentially revolutionizing acute myocardial infarction treatment by mitigating the risk of ischemic events in the critical post-event phase. [69,70,71,72]
Conclusion:
Research on the relationship between HDL and cardiovascular disease (CVD) has revealed that higher HDL levels may not be as straightforward as previously thought. Functional properties of HDL, such as cholesterol efflux and anti-inflammatory effects, may be more important predictors of cardiovascular health than absolute concentration. Genetic studies have identified specific variants related to HDL metabolism that may influence CVD risk independently of HDL levels. Clinicians should consider both HDL levels and HDL function when assessing cardiovascular risk. Further research is needed to understand the protective effects of HDL and develop targeted therapies for preventing and treating CVD.
References
-
Das P, Ingole N. Lipoproteins and their effects on the cardiovascular system. Cureus [Internet]. 2023 [cited 2024 Mar 6];15(11). Available from: http://dx.doi.org/10.7759/cureus.48865
View at Publisher | View at Google Scholar -
CDC. LDL and HDL cholesterol and triglycerides [Internet]. Centers for Disease Control and Prevention. 2023 [cited 2024 Mar 6]. Available from: https://www.cdc.gov/cholesterol/ldl_hdl.htm
View at Publisher | View at Google Scholar -
Barter PJ, Nicholls S, Rye K-A, Anantharamaiah GM, Navab M, Fogelman AM. Antiinflammatory properties of HDL. Circ Res [Internet]. 2004;95(8):764–72. Available from: http://dx.doi.org/10.1161/01.res.0000146094.59640.13
View at Publisher | View at Google Scholar -
Lu J, Han G, Liu X, Chen B, Peng K, Shi Y, et al. Association of high-density lipoprotein cholesterol with all-cause and cause-specific mortality in a Chinese population of 3.3 million adults: a prospective cohort study. Lancet Reg Health West Pac [Internet]. 2024;42(100874):100874. Available from: http://dx.doi.org/10.1016/j.lanwpc.2023.100874
View at Publisher | View at Google Scholar -
Prevention and treatment of high cholesterol (hyperlipidemia) [Internet]. www.heart.org. [cited 2024 Mar 6]. Available from: https://www.heart.org/en/health-topics/cholesterol/prevention-and-treatment-of-high-cholesterol-hyperlipidemia
View at Publisher | View at Google Scholar -
Ben-Aicha S, Badimon L, Vilahur G. Advances in HDL: Much more than lipid transporters. Int J Mol Sci [Internet]. 2020;21(3):732. Available from: http://dx.doi.org/10.3390/ijms21030732
View at Publisher | View at Google Scholar -
Remaley AT. Apolipoprotein A-II: Still second fiddle in high-density lipoprotein metabolism? Arterioscler Thromb Vasc Biol [Internet]. 2013;33(2):166–7. Available from: http://dx.doi.org/10.1161/atvbaha.112.300921
View at Publisher | View at Google Scholar -
Dornas W, Silva M. Modulation of the antioxidant enzyme paraoxonase-1 for protection against cardiovascular diseases. Nutr Metab Cardiovasc Dis [Internet]. 2024; Available from: http://dx.doi.org/10.1016/j.numecd.2024.04.005
View at Publisher | View at Google Scholar -
Nagao M, Nakajima H, Toh R, Hirata K-I, Ishida T. Cardioprotective effects of high-density lipoprotein beyond its anti-atherogenic action. J Atheroscler Thromb [Internet]. 2018 [cited 2024 Mar 6];25(10):985–93. Available from: http://dx.doi.org/10.5551/jat.rv17025
View at Publisher | View at Google Scholar -
von Eckardstein A, Nordestgaard BG, Remaley AT, Catapano AL. High-density lipoprotein revisited: biological functions and clinical relevance. Eur Heart J [Internet]. 2023 [cited 2024 Mar 6];44(16):1394–407. Available from: https://academic.oup.com/eurheartj/article/44/16/1394/6807326
View at Publisher | View at Google Scholar -
Marsche G, Heine GH, Stadler JT, Holzer M. Current understanding of the relationship of HDL composition, structure and function to their cardioprotective properties in chronic kidney disease. Biomolecules [Internet]. 2020;10(9):1348. Available from: http://dx.doi.org/10.3390/biom10091348
View at Publisher | View at Google Scholar -
Rousset X, Vaisman B, Amar M, Sethi AA, Remaley AT. Lecithin: cholesterol acyltransferase – from biochemistry to role in cardiovascular disease. Curr Opin Endocrinol Diabetes Obes [Internet]. 2009;16(2):163–71. Available from: http://dx.doi.org/10.1097/med.0b013e328329233b
View at Publisher | View at Google Scholar -
Sun Y, Li X. Cholesterol efflux mechanism revealed by structural analysis of human ABCA1 conformational states. Nat Cardiovasc Res [Internet]. 2022;1(3):238–45. Available from: http://dx.doi.org/10.1038/s44161-022-00022-y
View at Publisher | View at Google Scholar -
Temel RE, Trigatti B, DeMattos RB, Azhar S, Krieger M, Williams DL. Scavenger receptor class B, type I (SR-BI) is the major route for the delivery of high density lipoprotein cholesterol to the steroidogenic pathway in cultured mouse adrenocortical cells. Proc Natl Acad Sci U S A [Internet]. 1997;94(25):13600–5. Available from: http://dx.doi.org/10.1073/pnas.94.25.13600
View at Publisher | View at Google Scholar -
Dabravolski S, Orekhov NA, Melnichenko A, Sukhorukov VN, Popov MA, Orekhov A. Cholesteryl ester transfer protein (CETP) variations in relation to lipid profiles and cardiovascular diseases: An update. Curr Pharm Des [Internet]. 2024;30(10):742–56. Available from: http://dx.doi.org/10.2174/0113816128284695240219093612
View at Publisher | View at Google Scholar -
Sciencedirect.com. [cited 2024 Mar 6]. Available from: https://www.sciencedirect.com/science/article/pii/S0022227520356777n
View at Publisher | View at Google Scholar -
Nakanishi S, Vikstedt R, Söderlund S, Lee-Rueckert M, Hiukka A, Ehnholm C, et al. Serum, but not monocyte macrophage foam cells derived from low HDL-C subjects, displays reduced cholesterol efflux capacity. J Lipid Res [Internet]. 2009;50(2):183–92. Available from: http://dx.doi.org/10.1194/jlr.m800196-jlr200
View at Publisher | View at Google Scholar -
Ouimet M, Barrett TJ, Fisher EA. HDL and reverse cholesterol transport: Basic mechanisms and their roles in vascular health and disease. Circ Res [Internet]. 2019;124(10):1505–18. Available from: http://dx.doi.org/10.1161/circresaha.119.312617
View at Publisher | View at Google Scholar -
Robert J, Osto E, von Eckardstein A. The endothelium is both a target and a barrier of HDL’s protective functions. Cells [Internet]. 2021;10(5):1041. Available from: http://dx.doi.org/10.3390/cells10051041
View at Publisher | View at Google Scholar -
Frambach SJCM, de Haas R, Smeitink JAM, Rongen GA, Russel FGM, Schirris TJJ. Brothers in arms: ABCA1- and ABCG1-mediated cholesterol efflux as promising targets in cardiovascular disease treatment. Pharmacol Rev [Internet]. 2020;72(1):152–90. Available from: http://dx.doi.org/10.1124/pr.119.017897
View at Publisher | View at Google Scholar -
Wang S, Smith JD. ABCA1 and nascent HDL biogenesis. Biofactors [Internet]. 2014;40(6):547–54. Available from: http://dx.doi.org/10.1002/biof.1187
View at Publisher | View at Google Scholar -
Tall AR. Cholesterol efflux pathways and other potential mechanisms involved in the athero‐protective effect of high-density lipoproteins. J Intern Med [Internet]. 2008;263(3):256–73. Available from: http://dx.doi.org/10.1111/j.1365-2796.2007.01898.x
View at Publisher | View at Google Scholar -
Trigatti BL, Krieger M, Rigotti A. Influence of the HDL receptor SR-BI on lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol [Internet]. 2003;23(10):1732–8. Available from: http://dx.doi.org/10.1161/01.atv.0000091363.28501.84
View at Publisher | View at Google Scholar -
Lai S-J, Ohkawa R, Horiuchi Y, Kubota T, Tozuka M. Red blood cells participate in reverse cholesterol transport by mediating cholesterol efflux of high-density lipoprotein and apolipoprotein A-I from THP-1 macrophages. Biol Chem [Internet]. 2019;400(12):1593–602. Available from: http://dx.doi.org/10.1515/hsz-2019-0244
View at Publisher | View at Google Scholar -
Bura KS, Lord C, Marshall S, McDaniel A, Thomas G, Warrier M, et al. Intestinal SR-BI does not impact cholesterol absorption or transintestinal cholesterol efflux in mice. J Lipid Res [Internet]. 2013;54(6):1567–77. Available from: http://dx.doi.org/10.1194/jlr.m034454
View at Publisher | View at Google Scholar -
Säemann MD, Poglitsch M, Kopecky C, Haidinger M, Hörl WH, Weichhart T. The versatility of HDL: a crucial anti‐inflammatory regulator. Eur J Clin Invest [Internet]. 2010;40(12):1131–43. Available from: http://dx.doi.org/10.1111/j.1365-2362.2010.02361.x
View at Publisher | View at Google Scholar -
Singh V, Kaur R, Kumari P, Pasricha C, Singh R. ICAM-1 and VCAM-1: Gatekeepers in various inflammatory and cardiovascular disorders. Clin Chim Acta [Internet]. 2023;548(117487):117487. Available from: http://dx.doi.org/10.1016/j.cca.2023.117487
View at Publisher | View at Google Scholar -
Popko K, Gorska E, Stelmaszczyk-Emmel A, Plywaczewski R, Stoklosa A, Gorecka D, et al. Proinflammatory cytokines IL-6 and TNF-α and the development of inflammation in obese subjects. Eur J Med Res [Internet]. 2010;15(S2). Available from: http://dx.doi.org/10.1186/2047-783x-15-s2-120
View at Publisher | View at Google Scholar -
Higashi Y. Endothelial function in dyslipidemia: Roles of LDL-cholesterol, HDL-cholesterol and triglycerides. Cells [Internet]. 2023;12(9):1293. Available from: http://dx.doi.org/10.3390/cells12091293
View at Publisher | View at Google Scholar -
Bae EH, Kim IJ, Ma SK, Lee JU, Kim SW. Altered regulation of renal nitric oxide and atrial natriuretic peptide systems in lipopolysaccharide-induced kidney injury. Korean J Physiol Pharmacol [Internet]. 2011;15(5):273. Available from: http://dx.doi.org/10.4196/kjpp.2011.15.5.273
View at Publisher | View at Google Scholar -
Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by akt and MAP kinases. J Biol Chem [Internet]. 2003;278(11):9142–9. Available from: http://dx.doi.org/10.1074/jbc.m211394200
View at Publisher | View at Google Scholar -
Feig JE, Feig JL, Dangas GD. The role of HDL in plaque stabilization and regression: Basic mechanisms and clinical implications. Coron Artery Dis [Internet]. 2016;27(7):592–603. Available from: http://dx.doi.org/10.1097/mca.0000000000000408
View at Publisher | View at Google Scholar -
Di Bartolo BA, Psaltis PJ, Bursill CA, Nicholls SJ. Translating evidence of HDL and plaque regression. Arterioscler Thromb Vasc Biol [Internet]. 2018;38(9):1961–8. Available from: http://dx.doi.org/10.1161/atvbaha.118.307026
View at Publisher | View at Google Scholar -
Mineo C, Deguchi H, Griffin JH, Shaul PW. Endothelial and antithrombotic actions of HDL. Circ Res [Internet]. 2006;98(11):1352–64. Available from: http://dx.doi.org/10.1161/01.res.0000225982.01988.93
View at Publisher | View at Google Scholar -
Bhale AS, Venkataraman K. Leveraging knowledge of HDLs major protein ApoA1: Structure, function, mutations, and potential therapeutics. Biomed Pharmacother [Internet]. 2022;154(113634):113634. Available from: http://dx.doi.org/10.1016/j.biopha.2022.113634
View at Publisher | View at Google Scholar -
Ginsberg HN. Lipoprotein physiology. Endocrinol Metab Clin North Am [Internet]. 1998;27(3):503–19. Available from: http://dx.doi.org/10.1016/s0889-8529(05)70023-2
View at Publisher | View at Google Scholar -
Kobayashi J, Miyashita K, Nakajima K, Mabuchi H. Hepatic lipase: A comprehensive view of its role on plasma lipid and lipoprotein metabolism [Internet]. Jst.go.jp. [cited 2024 Sep 12]. Available from: https://www.jstage.jst.go.jp/article/jat/22/10/22_31617/_pdf
View at Publisher | View at Google Scholar -
Wang J, Xiao Q, Wang L, Wang Y, Wang D, Ding H. Role of ABCA1 in cardiovascular disease. J Pers Med [Internet]. 2022;12(6):1010. Available from: http://dx.doi.org/10.3390/jpm12061010
View at Publisher | View at Google Scholar -
Gracia-Rubio I, Martín C, Civeira F, Cenarro A. SR-B1, a key receptor involved in the progression of cardiovascular disease: A perspective from mice and human genetic studies. Biomedicines [Internet]. 2021;9(6):612. Available from: http://dx.doi.org/10.3390/biomedicines9060612
View at Publisher | View at Google Scholar -
Norata GD, Tsimikas S, Pirillo A, Catapano AL. Apolipoprotein C-III: From pathophysiology to pharmacology. Trends Pharmacol Sci [Internet]. 2015;36(10):675–87. Available from: http://dx.doi.org/10.1016/j.tips.2015.07.001
View at Publisher | View at Google Scholar -
Dewey FE, Gusarova V, Dunbar RL, O’Dushlaine C, Schurmann C, Gottesman O, et al. Genetic and pharmacologic inactivation of ANGPTL3 and cardiovascular disease. N Engl J Med [Internet]. 2017;377(3):211–21. Available from: http://dx.doi.org/10.1056/nejmoa1612790
View at Publisher | View at Google Scholar -
Zhou L, He M, Mo Z, Wu C, Yang H, Yu D, et al. A genome wide association study identifies common variants associated with lipid levels in the Chinese population. PLoS One [Internet]. 2013;8(12):e82420. Available from: http://dx.doi.org/10.1371/journal.pone.0082420
View at Publisher | View at Google Scholar -
Borén J, Packard CJ, Taskinen M-R. The roles of ApoC-III on the metabolism of triglyceride-rich lipoproteins in humans. Front Endocrinol (Lausanne) [Internet]. 2020;11. Available from: http://dx.doi.org/10.3389/fendo.2020.00474
View at Publisher | View at Google Scholar -
Nordestgaard LT, Christoffersen M, Lauridsen BK, Afzal S, Nordestgaard BG, Frikke-Schmidt R, et al. Long-term benefits and harms associated with genetic cholesteryl ester transfer protein deficiency in the general population. JAMA Cardiol [Internet]. 2022;7(1):55. Available from: http://dx.doi.org/10.1001/jamacardio.2021.3728
View at Publisher | View at Google Scholar -
Zambon A, Deeb SS, Pauletto P, Crepaldi G, Brunzell JD. Hepatic lipase: a marker for cardiovascular disease risk and response to therapy. Curr Opin Lipidol [Internet]. 2003;14(2):179–89. Available from: http://dx.doi.org/10.1097/00041433-200304000-00010
View at Publisher | View at Google Scholar -
Teigen M, Ølnes ÅS, Bjune K, Leren TP, Bogsrud MP, Strøm TB. Functional characterization of missense variants affecting the extracellular domains of ABCA1 using a fluorescence-based assay. J Lipid Res [Internet]. 2024;65(1):100482. Available from: http://dx.doi.org/10.1016/j.jlr.2023.100482
View at Publisher | View at Google Scholar -
Yang X, Sethi A, Yanek LR, Knapper C, Nordestgaard BG, Tybjærg-Hansen A, et al. SCARB1 gene variants are associated with the phenotype of combined high high-density lipoprotein cholesterol and high lipoprotein (a). Circ Cardiovasc Genet [Internet]. 2016;9(5):408–18. Available from: http://dx.doi.org/10.1161/circgenetics.116.001402
View at Publisher | View at Google Scholar -
Liu S, Liu J, Weng R, Gu X, Zhong Z. Apolipoprotein E gene polymorphism and the risk of cardiovascular disease and type 2 diabetes. BMC Cardiovasc Disord [Internet]. 2019;19(1). Available from: http://dx.doi.org/10.1186/s12872-019-1194-0
View at Publisher | View at Google Scholar -
León-Mimila P, Villamil-Ramírez H, Macías-Kauffer LR, Jacobo-Albavera L, López-Contreras BE, Posadas-Sánchez R, et al. Genome-wide association study identifies a functional SIDT2 variant associated with HDL-C (high-density lipoprotein cholesterol) levels and premature coronary artery disease. Arterioscler Thromb Vasc Biol [Internet]. 2021;41(9):2494–508. Available from: http://dx.doi.org/10.1161/atvbaha.120.315391
View at Publisher | View at Google Scholar -
Klos KLE, Sing CF, Boerwinkle E, Hamon SC, Rea TJ, Clark A, et al. Consistent effects of genes involved in reverse cholesterol transport on plasma lipid and apolipoprotein levels in CARDIA participants. Arterioscler Thromb Vasc Biol [Internet]. 2006;26(8):1828–36. Available from: http://dx.doi.org/10.1161/01.atv.0000231523.19199.45
View at Publisher | View at Google Scholar -
Ahmed HM, Miller M, Nasir K, McEvoy JW, Herrington D, Blumenthal RS, et al. Primary low level of high-density lipoprotein cholesterol and risks of coronary heart disease, cardiovascular disease, and death: Results from the multi-ethnic study of atherosclerosis. Am J Epidemiol [Internet]. 2016 [cited 2024 Mar 7];183(10):875–83. Available from: http://dx.doi.org/10.1093/aje/kwv305
View at Publisher | View at Google Scholar -
S. Mannu G, J.S. Zaman M, Gupta A, U. Rehman H, K. Myint P. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr Cardiol Rev [Internet]. 2013 [cited 2024 Mar 7];9(1):2–14. Available from: http://dx.doi.org/10.2174/157340313805076313
View at Publisher | View at Google Scholar -
S. Mannu G, J.S. Zaman M, Gupta A, U. Rehman H, K. Myint P. Evidence of lifestyle modification in the management of hypercholesterolemia. Curr Cardiol Rev [Internet]. 2013 [cited 2024 Mar 7];9(1):2–14. Available from: http://dx.doi.org/10.2174/157340313805076313
View at Publisher | View at Google Scholar -
Sirtori CR, Corsini A, Ruscica M. The role of high-density lipoprotein cholesterol in 2022. Curr Atheroscler Rep [Internet]. 2022 [cited 2024 Mar 7];24(5):365–77. Available from: http://dx.doi.org/10.1007/s11883-022-01012-y
View at Publisher | View at Google Scholar -
Upadhyay RK. Emerging risk biomarkers in cardiovascular diseases and disorders. J Lipids [Internet]. 2015 [cited 2024 Mar 7];2015:1–50. Available from: http://dx.doi.org/10.1155/2015/971453
View at Publisher | View at Google Scholar -
Soppert J, Lehrke M, Marx N, Jankowski J, Noels H. Lipoproteins and lipids in cardiovascular disease: from mechanistic insights to therapeutic targeting. Adv Drug Deliv Rev [Internet]. 2020;159:4–33. Available from: http://dx.doi.org/10.1016/j.addr.2020.07.019
View at Publisher | View at Google Scholar -
Alankrita R, Marsche G. A current update on the role of HDL-based nanomedicine in targeting macrophages in cardiovascular disease. Pharmaceutics [Internet]. 2023 [cited 2024 Mar 7];15(5):1504. Available from: https://www.mdpi.com/1999-4923/15/5/1504
View at Publisher | View at Google Scholar -
Ali KM, Wonnerth A, Huber K, Wojta J. Cardiovascular disease risk reduction by raising HDL cholesterol – current therapies and future opportunities. Br J Pharmacol [Internet]. 2012 [cited 2024 Mar 7];167(6):1177–94. Available from: http://dx.doi.org/10.1111/j.1476-5381.2012.02081.x
View at Publisher | View at Google Scholar -
Kardassis D, Thymiakou E, Chroni A. Genetics and regulation of HDL metabolism. Biochim Biophys Acta Mol Cell Biol Lipids [Internet]. 2022;1867(1):159060. Available from: http://dx.doi.org/10.1016/j.bbalip.2021.159060
View at Publisher | View at Google Scholar -
Vourakis M, Mayer G, Rousseau G. The role of gut Microbiota on cholesterol metabolism in atherosclerosis. Int J Mol Sci [Internet]. 2021 [cited 2024 Mar 7];22(15):8074. Available from: http://dx.doi.org/10.3390/ijms22158074
View at Publisher | View at Google Scholar -
Brown EM, Clardy J, Xavier RJ. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe [Internet]. 2023;31(2):173–86. Available from: http://dx.doi.org/10.1016/j.chom.2023.01.009
View at Publisher | View at Google Scholar -
Shi Y, Zhang H, Huang S, Yin L, Wang F, Luo P, et al. Epigenetic regulation in cardiovascular disease: mechanisms and advances in clinical trials. Signal Transduct Target Ther [Internet]. 2022 [cited 2024 Mar 7];7(1). Available from: http://dx.doi.org/10.1038/s41392-022-01055-2
View at Publisher | View at Google Scholar -
Luthi AJ, Patel PC, Ko CH, Mutharasan RK, Mirkin CA, Thaxton CS. Nanotechnology for synthetic high-density lipoproteins. Trends Mol Med [Internet]. 2010 [cited 2024 Mar 7];16(12):553–60. Available from: http://dx.doi.org/10.1016/j.molmed.2010.10.006
View at Publisher | View at Google Scholar -
Alankrita R, Marsche G. A current update on the role of HDL-based nanomedicine in targeting macrophages in cardiovascular disease. Pharmaceutics [Internet]. 2023 [cited 2024 Mar 7];15(5):1504. Available from: https://www.mdpi.com/1999-4923/15/5/1504
View at Publisher | View at Google Scholar -
Booz GW, Altara R, Zouein FA. Editorial: Immunomodulatory approaches in cardiovascular diseases. Front Cardiovasc Med [Internet]. 2022 [cited 2024 Mar 7];9. Available from: http://dx.doi.org/10.3389/fcvm.2022.873452
View at Publisher | View at Google Scholar -
Jha BK, Sherpa ML, Imran M, Mohammed Y, Jha LA, Paudel KR, et al. Progress in understanding metabolic syndrome and knowledge of its complex pathophysiology. Diabetology [Internet]. 2023 [cited 2024 Mar 7];4(2):134–59. Available from: https://www.mdpi.com/2673-4540/4/2/15
View at Publisher | View at Google Scholar -
Researchgate.net. [cited 2024 Mar 7]. Available from: https://www.researchgate.net/publication/239948836_HDL_Atherosclerosis_and_Emerging_Therapies
View at Publisher | View at Google Scholar -
Hewing B, Fisher EA. Rationale for cholesteryl ester transfer protein inhibition. Curr Opin Lipidol [Internet]. 2012 [cited 2024 Mar 7];23(4):372–6. Available from: http://dx.doi.org/10.1097/mol.0b013e328353ef1d
View at Publisher | View at Google Scholar -
Alankrita R, Marsche G. A current update on the role of HDL-based nanomedicine in targeting macrophages in cardiovascular disease. Pharmaceutics [Internet]. 2023 [cited 2024 Mar 7];15(5):1504. Available from: https://www.mdpi.com/1999-4923/15/5/1504
View at Publisher | View at Google Scholar -
Liu C, Dhindsa D, Almuwaqqat Z, Ko Y-A, Mehta A, Alkhoder AA, et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiol [Internet]. 2022 [cited 2024 Mar 7];7(7):672. Available from: https://jamanetwork.com/journals/jamacardiology/fullarticle/2792282
View at Publisher | View at Google Scholar -
Güleç’ [’sadi, Erol’] ’cetin. High-density lipoprotein cholesterol and risk of cardiovascular disease [Internet]. Escardio.org. [cited 2024 Mar 7]. Available from: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-19/high-density-lipoprotein-cholesterol-and-risk-of-cardiovascular-disease
View at Publisher | View at Google Scholar -
Kim HJ, Jeong S, Oh YH, Park SJ, Cho Y, Park SM. Changes in high-density lipoprotein cholesterol with risk of Cardiovascular Disease among initially high-density lipoprotein-high participants. Cardiovasc Diabetol [Internet]. 2023;22(1). Available from: http://dx.doi.org/10.1186/s12933-023-01805-8
View at Publisher | View at Google Scholar






