Research Article | DOI: https://doi.org/CCSRR-RA-25-019.
How Nitroxide-Restricted Endothelial Function Contributes to The Development of Acute Lung Abscess
Abstract
Background: Among the leading causes of infection and mortality worldwide, purulent- destructive lung diseases continue to rank high. Impairment of this organ's barrier-filtration function, based on endothelial dysfunction, may be connected with the key to infection development in acute lung abscesses.
Methods: sing a rat model of acute lung abscess, 32 Chinchilla rabbits were used in the research. Blood samples taken at the point of entry and departure from the lungs are examined for markers such as nitrates, nitrites, peroxynitrite, NO-synthase, and von Willebrand factor.
Conclusion: In addition to improving local blood circulation and inhibiting platelet aggregation, the nitric oxide generated by iNOS activation is meant to provide non-specific protection to the body against a broad spectrum of harmful chemicals. Nevertheless, these alterations never materialize. Peroxynitrite, which is pathogenic and exacerbates the process of endothelial dysfunction, is primarily responsible for this. A compensated or decompensated state describes the changes in parameters of the nitroxidergic control of the endothelial system in the lungs. Everything is occurring naturally, according to established correlations in the nitroxidergic regulatory system's indications.
Introduction
Clinicians have begun to more frequently identify patients with purulent-destructive lung illnesses as having what is known as "multiple organ failure syndrome"—a combination of disorders affecting the function of several organs and systems[1]. Significantly, once the pathogenic process has broken past the lung endothelium barrier, it tends to spread throughout the body, eventually causing septic shock and the development of this syndrome[2],[3],[4]
We know that R. Furschgott was the one who found nitric oxide (NO)[5, 6]. Because it is the most stable free radical, forms continuously in the vascular endothelium of many organs, ensures sufficient tissue perfusion and blood pressure, protects the myocardium from arrhythmia, the lungs from hypoxia, and mitigates the harmful effects of thromboxane A2, NO is of particular interest.4 Another function of NO is to control mast cell histamine release and to reduce inflammation in blood vessel walls [7],[8], When NO reacts with oxidants, it creates peroxynitrite, a molecule that is extremely hazardous[9].
In 1887, I.P. Pavlov observed the distinctive fibrinolytic characteristics of lung tissue, which led him to speculate that the lungs may play a role in the metabolism of several physiologically active chemicals. The idea that the lungs regulate the blood content of several chemicals was proposed by Julius Comro in 1953. This idea was supported by Nagaishi C.'s first investigations on vasoactive chemicals, and the issue has received more attention due to the large number of comparable papers published in recent years. The concept of the lungs' metabolic activity, which aims to regulate the levels of many chemicals in the systemic circulation, gave rise to subsequent phrases such as "endogenous lung filter" and "lung barrier," among others. One of the processes that is not fully understood is the involvement of nitroxidergic modulation of the endothelium system in purulent- destructive illnesses of the lungs. This is a crucial link in the pathophysiology of the process, according to many studies[10], [11].
We set out to investigate the pathophysiology of acute lung abscess by identifying the function and location of nitroxidergic control of the endothelium system.
Materials and Methods
Experimental experiments were conducted on 32 healthy, disease-free, female and male Chinchilla rabbits (weighing 2.0-2.5 kg) who were quarantined in a vivarium for 10 days. The animals were housed in a typical environment. Standard diets were used for animal nutrition. Our approach was used to simulate an acute lung abscess in animals by injecting a 5% rabbit autocal solution into their lung tissue at a rate of 1 ml per kg of animal weight.3 The animals showed signs of an acute lung abscess during the course of the following seven days, which were corroborated by x-ray investigations.
In order to study the endothelium system in the lungs, researchers measured nitrates (NO2), nitrites (NO3), and peroxynitrite (ONOO-) in blood samples. They also measured the enzyme activity of NO synthases (eNOS and iNOS) using the Griess technique modified.11 Taking into account the dilution that occurs during deproteinization, the nitrite concentration was determined using the equation of the calibration curve. Using a 520 nm wavelength, an SF-46 spectrophotometer was used to determine optical density.
A package of reagents from Human (Germany) was used to study the von Willebrand factor (VWF) using a Humaclot DUO closed-type automated analyzer (Germany).
In addition to the usual ways of analyzing the results, which involve finding the average metabolic product content and the average error (M±m) in all the animals tested, for every experiment we calculated the venous-arterial difference, which is the difference between the substrates in the inflowing (mixed venous blood) and flowing (arterial blood) blood. This blood was accessed through a catheter placed at the mouth of the right atrium and delivered to the lungs. A positive value for the venous-arterial difference would indicate that the substrate is being synthesized in the lungs; a negative value would indicate that the substrates being studied are being consumed or rendered inactive in the lungs; and a zero value would indicate that the ratio of the lungs to the test substrate is neutral. On days 1, 3, 7, and 14 of the diseased process's progression, a comparable study procedure was conducted on animals.
Between 2020 and 2021, all of the study was place at the CMH Multan main research facility.
Results and Discussion
varying blood samples had significantly varying levels of NO content (p<0>
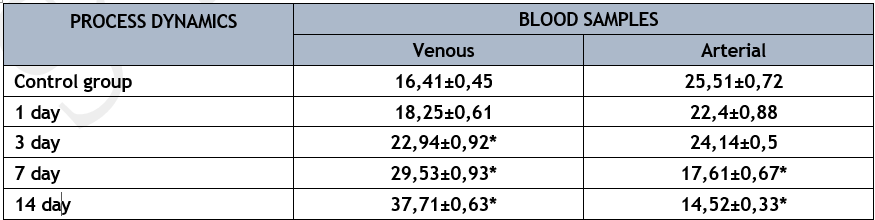
* р<0>
By simulating an acute lung abscess in the lab, we found that NO levels in the mixed venous blood sample taken near the lung's entry rose over time whereas they fell in the arterial blood sample. On the first day of the pathological process's growth, the venous-arterial difference declined by more than 2 times; on the third day, it decreased by 9 times; and throughout the trial, it remained at a positive value, much like the control group. Nevertheless, the venous-arterial difference began to decrease in the arterial blood flow system, and the amount of this metabolite began to rise again on the seventh day of the trials. It appears that unstable arterial pressure, caused by low NO levels in the arterial blood sample, led to the development of septic shock and the spread of infection.
Along with NO generation in the lungs' endothelial system, the eNOS enzyme activity increased from 2.82±0.14 µmol/min/l in mixed venous blood to 5.63±0.17 µmol/min/l (p<0>
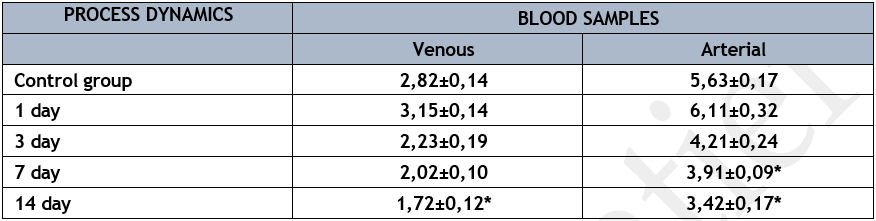
* р<0>
This enzyme's activity rose in arterial and mixed venous blood samples on the first day of acute lung abscess modeling. However, as compared to the control group of studies, there was no statistically significant increase in this enzyme's activity in the lung endothelium system. The enzyme's activity steadily declined in successive periods, peaked on the fourteenth day of the diseased phase in both arterial and mixed venous blood samples. It is worth mentioning that the endothelial system of the lungs showed a drop in NOS creation alongside the decrease in NOS activity in mixed venous and arterial blood samples. That is, endothelial dysfunction, as shown by the low activity of the enzyme under research, developed alongside the reproduction of the experimental model of acute lung abscess. Simultaneously, during the first phases of developing an animal model of acute lung abscess, a notable disruption of the nitroxidergic regulatory system was seen.
Mixed venous blood samples showed considerably greater iNOS isoenzyme activity (Table 3) compared to arterial blood samples in the control set of trials (p<0>
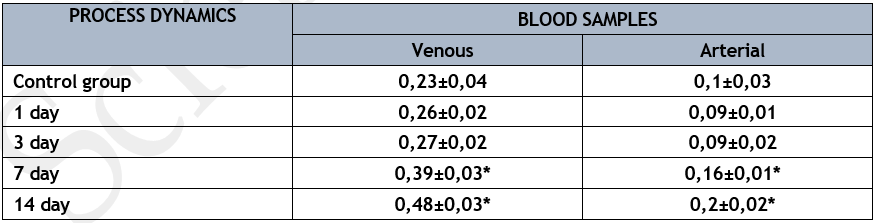
* р<0>
Compared to the control group, the arterial blood sample showed a relatively stable activity of the iNOS isoenzyme on the first three days of the pathological process's development, which was accompanied by an increase in its venous-arterial difference during this time (p<0>
The level of the iNOS isoenzyme's activity in the arterial blood sample at the exit from the lungs significantly increased compared to the control group, even though there was a twofold increase in the venous-arterial difference on the 7th and 14th days of the pathological process's development (p<0>
In the series of control studies, the amount of ONOO-decreased from 1.51±0.04 µmol/l in mixed venous blood entering the lungs to 0.60±0.04 µmol/l in the arterial blood sample leaving the lungs (p<0>
An uptick in ONOO-in mixed venous blood, seemingly emanating from a purulent-septic focus, was noted during the early phases of acute lung abscess reproduction in the experimental model.
At this time, the endothelium system of the lungs was making more use of this substrate, which helped maintain its concentration in the arterial blood within normal limits since the lungs were actively performing their barrier-filtration function.
The concentration of ONOO- in mixed venous blood increased as the experimental model of acute lung abscess progressed, reaching its highest value on the fourteenth day of the studies.
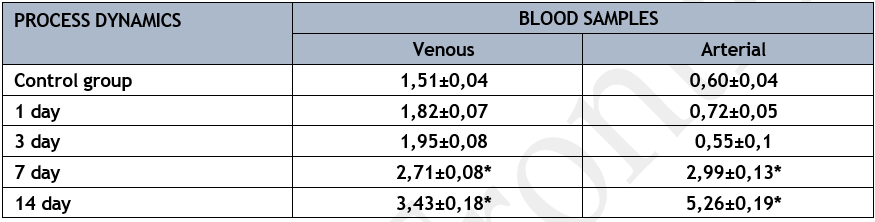
* р<0>
The concentration of this product, which is harmful to endothelial cell membrane structures and contributes to their destruction, increased significantly in the arterial blood sample over these study periods. At this stage, the lungs go from being an organ that just takes in oxygen to one that actively helps make new oxygen by releasing ONOO- into the arterial bed. As the pathogenic process begins to develop, damage to the lung endothelium system happens between days 7 and 14, setting in motion the chain reaction that eventually leads to the septic process spreading throughout the body. The blood level of VWF is one of the markers for endothelial cell injury (Table 5). The participation of this product in the activity of the lung's barrier-filtration function was demonstrated by its use in the control series of studies. In the early days of acute lung abscess modeling, this substrate's concentration in mixed venous blood near the lung's entry was significantly higher. Simultaneously, on the first and third days of the pathological process's development, there was a 1.8-fold rise in the venous-arterial difference compared to the control set of trials, indicating a substantial improvement in the lungs' functional capacity (p<0>
On the first day of the pathological process, the amount of VWF in the arterial blood sample was 21 times higher than the control values; on the third day, it was 48 times higher. It seems that this occurred as a result of the nitroxidergic system's incorporation of endothelial dysfunction processes and the start of disseminated intravascular coagulation. This substrate's level in the arterial blood sample and mixed venous blood sample peaked on the fourteenth day of the pathological process, as a result of progressive endothelial dysfunction in the subsequent phases of the experimental model of acute lung abscess's development.
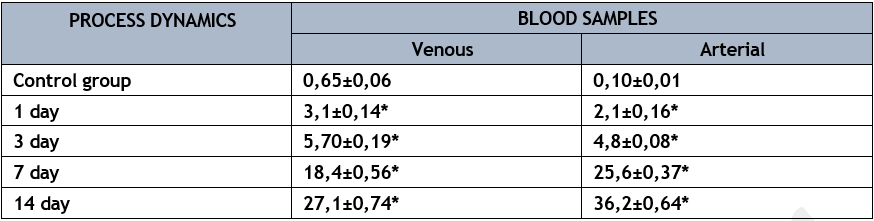
* р<0>
Because the endothelium system contributed to the production of this product in the lungs, the venous-arterial difference in these experimental parameters was defined by a "plus" number. This data proves without a doubt that endothelial dysfunction is worsening in the lungs. On the seventh day of the pathogenic process, the level of the venous-arterial difference climbed to 7.20±1.15 µmol/l, and on the fourteenth day of the sickness, it increased to 9.10±1.66 µmol/l. 16.5 times and 13.
Rapid interaction between NO and partly decreased oxygen leads to the creation of peroxynitrite, which damages endothelium system cells, most noticeably the lungs. The elimination of NO from the arterial wall and alveolocyte surfaces is aided by this process[12].
The alveolar epithelium and lung surfactant system are vulnerable to peroxynitrite, a powerful oxidant.1 It contributes to endotoxemia by destroying membrane proteins and lipids, damaging the endothelium, and increasing platelet aggregation. In cases of acute lung damage, its production was shown to be elevated[13].
Research on the nitroxidergic regulatory system's isoenzyme content (iNOS) revealed more specific alterations than those of eNOS. You are aware that cytokines, lipopolysaccharides, and other cellular forms depending on pathology trigger the activation of normally functioning cells, which in turn create this enzyme[14].
The nitric oxide that is generated when iNOS is activated has many functions: it protects the body from various harmful substances, prevents platelets from clumping together, and enhances blood circulation in certain areas. But these alterations never take place. Since peroxynitrite is pathogenic, it exacerbates the already-present process of endothelial dysfunction and is hence primarily responsible for this direction. A significant component of these alterations is the elevated activity of iNOS, which is induced by cytokines that promote inflammation.16 If the inflammatory process damages the lungs to a certain extent, then the systemic circulation will produce cytokines to varying degrees.
The impact of NO and peroxynitrite on elastase is well-known to be the foundation of acute lung damage. A higher concentration of iNOS and other cytokines was found in bronchoalveolar fluid from individuals with acute lung injury syndrome, according to Kobayashi et al.[15].
Conclusion
Accordingly, there is a staging system for the kind of alterations in the markers of nitroxidergic control of the lung endothelium system. The lungs adapted to variations in the concentrations of certain substrates by increasing or lowering their barrier-filtration functional activity in the early stages of the diseased process. Changes in the arterial blood composition near the departure of the lungs were a reflection of the suppression of this functional activity, which was aided by the advancement of the inflammatory process into sepsis.
Based on certain relationships between the indicators of the nitroxidergic system of regulation, we believe that the deviation in the physiological parameters of the lung's barrier-filtration function, as stated in the dynamics of the development of the experimental model of acute lung abscess compared to the control series of experiments, is natural.
Source of funding
Self.
Conflict of Interest
No
References
-
Amunugama, K., D.P. Pike, and D.A. Ford, E. coli strain-dependent lipid alterations in cocultures with endothelial cells and neutrophils modeling sepsis. Frontiers in physiology, 2022. 13: p. 980460.
View at Publisher | View at Google Scholar -
Atakov, S., et al., Difficult aspects of treatments patients with acute lung abscesses who survived COVID-19. 2022.
View at Publisher | View at Google Scholar -
Mazhar, M.W., et al., Detection of SARS-CoV-2 by using real-time PCR nasopharyngeal swabs in suspected patients and their clinical medication. Sensors International, 2022. 3: p. 100148.
View at Publisher | View at Google Scholar -
Mazhar, M.W., Omicron BA 2: Vaccine Construction with MHC Class-I, MHC Class-II by Using Bioinformatics Tools. Archives of Immunology Research and Therapy, 2024. 3(1).
View at Publisher | View at Google Scholar -
Bobokulova, S.A. and A. Okhunov, Acute Purulent-Destructive Lung Diseases as Consequences of Endotheliitis after COVID 19. 2022.
View at Publisher | View at Google Scholar -
Li, Z., et al., In vivo evaluation of a lipopolysaccharide-induced ear vascular leakage model in mice using photoacoustic microscopy. Biomedical optics express, 2022. 13(9): p. 4802-4816.
View at Publisher | View at Google Scholar -
Bredt, D.S., et al., Cloned and expressed nitric oxide synthase structurally resembles cytochrome P- 450 reductase. Nature, 1991. 351(6329): p. 714-718.
View at Publisher | View at Google Scholar -
MAZHAR, M.W., et al., Severe acute respiratory syndrome (Saras Covid-2): a comprehensive review. Quantum Journal of Medical and Health Sciences, 2021. 1(4): p. 25-33.
View at Publisher | View at Google Scholar -
Brovkovych, V., et al., Augmented inducible nitric oxide synthase expression and increased NO production reduce sepsis-induced lung injury and mortality in myeloperoxidase-null mice. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2008. 295(1): p. L96-L103.
View at Publisher | View at Google Scholar -
Okhunov, A., et al., Treatment of acute lung abscesses considering their non-respiratory function in patients with diabetes. Indian Journal of Forensic Medicine and Toxicology, 2020. 14(4): p. 7465- 7469.
View at Publisher | View at Google Scholar -
Okhunov, A. and A. Kasymov, Some pathogenic aspects of changes in non-respiratory function of the lungs in sepsis. Likars' ka Sprava, 2006(7): p. 45-47.
View at Publisher | View at Google Scholar -
Blaylock, M.G., et al., The effect of nitric oxide and peroxynitrite on apoptosis in human polymorphonuclear leukocytes. Free Radical Biology and Medicine, 1998. 25(6): p. 748-752.
View at Publisher | View at Google Scholar -
Охунов, А.О. and Ш.А. Бобокулова, The role and place of nitroxidergic regulation of the endothelial system in the pathogenesis of acute lung abscess. 2022.
View at Publisher | View at Google Scholar -
Meldrum, D.R., et al., Nitric oxide downregulates lung macrophage inflammatory cytokine production. The Annals of thoracic surgery, 1998. 66(2): p. 313-317.
View at Publisher | View at Google Scholar -
Kobayashi, A., et al., Expression of inducible nitric oxide synthase and inflammatory cytokines in alveolar macrophages of ARDS following sepsis. Chest, 1998. 113(6): p. 1632-1639.
View at Publisher | View at Google Scholar






