Research Article | DOI: https://doi.org/JCCRI-RA-25-015.
Antibacterial Effect of Tetrapleura Tetraptera (dawo) and Monodora Myristica (ehuru) Plantextracts on Staphylococcus Aureus and Escherichia Coli.
Abstract
The study was conducted to evaluate the antibacterial effect of medicinal plant extracts Tetrapleura tetraptera and Monodora myristica against specific bacteria pathogens Escherichia coli and Staphylococcus aureus.The plants were extracted using two types of extraction methods namely hot aqueous and ethanol extracts.Antibacterial testing was determined using well-in-agar diffusion method.Antibacterial activity was evaluated by measuring the inhibitory zone of the T.terapteraand M.myristica extract against the tested bacteria pathogens.Phytochemical screening was conducted on both T.tetrapteraand M.myristica extracts. The results for phytochemical screening forM.myristica and T.tetraptera showed presence of alkaloids, phenols, tanins, flavoids, glycosides, saponins and terpenoids in different amounts in each of the extracts. Zone of inhibition results produced by M.myrstica , hot aquoes extract showed 11mm,4mm, at 10mg and 7.5mg concentrations againtsS.areus then 17mm,13mm,9mm at 10mg,7.5mg and 5.0mg against E.coli. The ethanol extract showed 15mm, 8mm,5mm at 10mg,7.5mg and 5.0mg concentrations against E.coli then 19mm, 12mm, 9mm and 4mm at all concentrations against S.aureus. Zone of inhibition results produced by T.tetraptera, hot aqueous extract showed 13mm, 8mm, 3mm at 10mg, 7.5mg and 5.0mg concentrations against E.coli then 19mm, 15mm, 1mm and 5mm at all concentrations against S.aureus. The ethanol extract showed 21mm, 11mm, 8mm, 3mm at all concentrations against E. coli then 22mm, 14mm, 12mm,5mm at all concentrations against S.aureus. Analysis of result showed that ethanol extracts were more active against the two bacteria pathogens of interest at all concentrations whereas the hot aqueous extract was more effective at 10mg, 7.5mg and 5.0mg concentrations. It also showed that the ethanol extract of T.tetraptera was most effective plant extract against the two bacteria pathogens showing higher zones of inhibition. However, when the results of the plant extracts were compared to the control used in the study which is ciprofloxacin, it had higher zones of inhibition (24mm) than that produced by the plant extracts. Due to the observed antibacterial effect of the plant extracts, they can be further studied for the development of alternative therapy to the use of antibiotic .
Introduction
In an era shadowed by the looming threat of antibiotic resistance, the exploration of alternative antimicrobial solutions has emerged as a critical endeavor. Antimicrobial agents constitute a diverse group of compounds designed to inhibit microorganism growth or destroy them, with antibiotics representing a crucial subset specifically targeting bacteria [1]. The historical use of medicinal plants for therapeutic purposes spans centuries and is deeply rooted in traditional medical systems such as Ayurveda, Traditional Chinese Medicine and Indigenous knowledge systems. The advent of synthetic drugs in the 20th century temporarily shifted the focus away from natural sources. However, the rise of antibiotic-resistant strains has reignited interest in traditional remedies, prompting scientific investigations into the antibacterial properties of medicinal plants [2]. Recognizing the limitations and challenges associated with synthetic antibiotics, there is a renewed interest in exploring alternative sources, such as medicinal plants, known for their rich bioactive compound content. These bioactive molecules can disrupt bacterial cell walls, inhibit essential enzymes, or interfere with bacterial protein synthesis, rendering them potential candidates for combating bacterial infections. Recent studies have highlighted the potential of medicinal plant extracts as effective antibacterial agents [3]. Furthermore, plants with naturally available phytochemicals provide resistance against different diseases [4]. Phytochemical such as proteins, Chlorophyll, and common sugars are considered as primary compounds, and alkaloids, terpenoid, and phenolic are secondary compounds [5]. This project focuses on specific bacteria, such as Staphylococcus aureus and Escherichia coli, prevalent in human infections. Staphylococcus aureus, a Gram-positive bacterium, is infamous for causing skin infections, while Escherichia coli, a Gram-negative bacterium, is commonly associated with urinary tract and gastrointestinal infections. This deliberate selection enables a comprehensive evaluation of the efficacy of medicinal plant extracts against both Gram-positive and Gram-negative bacterial strains [7]. Evaluating medicinal plant extracts against these strains provides a comprehensive assessment of their potential efficacy in the face of antibiotic-resistant pathogens. This testing of medicinal extracts against pathogens is known as antibiotic sensitivity testing. Antibiotic sensitivity testing or antibiotic susceptibility testing is the measurement of the susceptibility of bacteria to antibiotics. It is used because bacteria may have resistance to some antibiotics [8]. Sensitivity testing results can allow a clinician to change the choice of antibiotics from empiric therapy, which is when an antibiotic is selected based on clinical suspicion about the site of an infection and common causative bacteria, to directed therapy, in which the choice of antibiotic is based on knowledge of the organism and its sensitivities [9].
The assessment of the antibacterial effects of medicinal plant extracts on specific bacteria is a crucial scientific pursuit rooted in the need for innovative solutions to combat microbial threats. This research aims to scrutinize the potential therapeutic efficacy of natural compounds derived from medicinal plants against targeted bacterial strains. First and foremost, medicinal plants have a rich history of serving as reservoirs for diverse bioactive compounds, many of which exhibit pronounced antimicrobial properties. By systematically analyzing the antibacterial effects of these plant extracts, researchers can identify novel agents that may offer effective alternatives or supplements to existing antibiotics [10].
Moreover, the specificity of the evaluation, focusing on particular bacterial strains, adds precision to the study. This approach allows for a nuanced understanding of the spectrum of antibacterial activity, enabling researchers. In an era of increasing antibiotic resistance, such targeted investigations are paramount for developing therapies that can address the evolving landscape of bacterial threats
Materials And Methods
STUDY AREA
The study was carried out in Aba, Abia State, Nigeria. Rhema university is located along Aba -Owerri road, Aba, Abia state. Abia State (Igbo: ȮhaAbia) is a state in the South-East geopolitical zone of Nigeria, it is bordered to the northwest by Anambra State and northeast by the states of Enugu, and Ebonyi, Imo State to the west, Cross River State to the east, Akwa Ibom State to the southeast, and Rivers State to the south. Aba is a city in the southeast of Nigeria and the commercial center of Abia State. Upon the creation of Abia state in 1991, Aba was divided into two local government areas; Aba South and Aba North. The indigenous people of Aba are the Ngwa. Aba is well known for its craftsmen and also the most populous city in the South Eastern Nigeria. As of 2016, Aba had an estimated population of 2,534,265. The state's slogan is "God's own State”
The wet season in Aba is warm and overcast while the dry season is hot and mainly cloudy, and it is very hot all year round. Over a period of the year, the temperature typically changes from 68 °F to 88 °F and is rarely below 61 °F or over 91 °F
Bacterial Collection
S.aureus and E.coli were isolated from wound and urinary samples in Rhema University Teaching Hospital(R.U.T.H) were used in this study.
Collection Of Medicinal Plants
The plant materials used in this study consisted of Tetrapleura tetraptera and Monodora myristica. The selected medicinal plants were collected from market (Afule market) in Aba, Abia state.
Processing Of Medicinal Plant
Cleaning
Cleaning of Plants After plants collection they have to be cleaned properly. The cleaning process may involve the following steps. Cleaning, washing, peeling or stripping leaves from stems. Cleaning has to be done by hands in order to get better results.
Drying
The main purpose of drying is to remove the water content from plants so that the palnts can be stored. Plants have to be dried immediately as soon as the plants collection or this will lead to spoilage of plant materials. The drying was done by artificial method. Artificial drying can be done with the help of artificial driers. This process will reduce the drying time to several hours or minutes. The common method that is followed in drying medicinal plants is warm-air drying. In this process plants are placed in the plates of drier on which warm air is blown.
Extraction Of Medicinal Plant
The method used is maceration. In maceration (for fluid extract), whole or coarsely powdered plant is kept in contact with the solvent in a stoppered container for a defined period with frequent agitation until soluble matter is dissolved.
The solvents used were water and ethanol.
Hot aqueous extraction process
A total of 12g of dried plant powder was weighed and soaked in 150ml of hot distilled water in a 250ml conical flask and boiled for 30 minutes before keeping it at room temperature for 24hours. It was filtered using sterile Whatman No.1 filter paper into new sterile 250ml conical flask. This was done seperately for each plant.
Ethanol extraction process
A total of 15g of dried plant powder was weighed and soaked in 200ml of ethanol in a 250ml conical flask for 24hours. It was filtered using sterile Whatman No.1 filter paper into new sterile 250ml conical flask. This was done separately for each plant.
PHYTOCHEMICAL ANALYSIS OF MEDICINAL PLANT
The ethanol and aqueous extracts obtained from the extraction process were analyzed for different phyto-constituents such as saponin, flavonoid, alkaloids, tannins, phenols, glycosides and terpenoids which were present in these by the method of qualitative phytochemical analysis.
Test for Alkaloids
Using Mayer’ s test To a few ml of plant sample extract, two drops of Mayer‟s reagent are added along the sides of test tube. Appearance of white creamy precipitate indicates the presence of alkaloids.
Test for Glycosides
The extract is hydrolyzed with concentrated hydrochloric acid for 2 hours on a water bath, filtered and the hydrolysate is subjected to the following tests.
Using Borntrager’s test To 2 ml of filtered hydrolysate, 3 ml of choloroform is added and shaken, choloroform layer is separated and 10% ammomia solution is added to it. Pink colour indicates presence of glycosides.
Test for Phenolic compounds
Using Ferric Chloride test method, the extract (50 mg) is dissolved in 5 ml of distilled water. To this few drop of neutral 5?rric chloride solution are added. A dark green colour indicates the presence of phenolic compound.
Test for saponins
Extract was placed in a test tube and shaken vigorously. The formation of stable foam was taken as an indication for the presence of saponins.
Test for terpenoids
Extract was mixed with 2ml of chloroform. Then 2ml of concentrated sulfuric acid was added carefully and shaken gently. A reddish-brown coloration is formed to show positive results.
Test for tannins
To 5ml of extract few drops of neutral 5?rric chloride solution was added, the production of darkgreencolour indicates the presence of tannins
Cultivation Of Microorganisms
Fifteen (15) culture media plates were prepared, allowed to cool and dried in the hot air oven. Quality control was carried out by incubating one of each agar plate for 24hours at 37°C and it was examined for growth. When there is no growth it indicates a good media preparation.
After the serial dilution, the samples 10-1 10-3 and 10-5 were inoculated on Macconkey, Eosin Methylene Blue (EMB) agar, Salmonella Shigella Agar (SSA), and Manitol salt agar. Spread plate method was used for culturing. 1ml of each dilution was pipetted and plated on the different agar then a hockey stick was used to spread it on the agar surface. The agar plate was then inverted and incubated at 37ºc for 24 hours.
The plates with growth were counted and nutrient agar was prepared for sub culturing discrete bacteria colonies and pure colonies. This was done in a sterile environment using spirit lamp and sterile wire loop. After sub culturing, these plates were incubated at 37°C for 24 hours. It was examined for growth the following day. The bacteria growths were isolated by purifying the growths in a nutrient agar slant in a McCartney bottle.
Identification Of Bacteria
The identification of these isolates after incubation was done by using some characteristics peculiar to them which include colonial appearance, Morphological characteristics, Gram staining and Biochemical test. The isolates were determined by studying the plates for size, shape, color, odour, colony forming unit count, consistency, fermentation, elevation, edge and pigmentation. The results were taken and recorded.
Gram staining reaction
Principle: Gram staining reaction differentiates gram negative bacteria from gram positive bacteria due to the differences in their cell wall, the gram-positive form possesses high content of peptidoglycan layer which enable them to retain the primary stain, crystal violet and to resist decolourisation by an acetone while the gram negative possesses low amount of peptidoglycan and readily decolourised by acetone and as a result, they pick up the colour of the counterstain.
Staining Procedure
With the aid of a sterile wireloop, the isolate was smeared onto a clean clear grease free slide and allowed to air dry. The primary stain, crystal violet was poured on the smear and allowed to stain for 60 seconds. It was washed in gentle running tap water for 5 seconds, covered with Lugol's iodine and rinsed in distilled water. The stain was decolourised using 95% alcohol, rinsed with tap water and then counterstained using neutral red for 60 seconds. The smear was finally rinsed in clean tap water, air dried and then a drop of immersion oil was placed on the stained slide. It was viewed under the microscope using 100X objective lens.
Biochemical Tests
Catalase Test
This test is used to differentiate those bacteria that produce the enzyme catalase such as staphylococci, from non-catalase producing bacteria such as streptococci.
Principle: Catalase acts as a catalyst in the breakdown of hydrogen peroxide to oxygen and water. An organism is tested for catalase production by bringing it into contact with hydrogen peroxide. Bubbles of oxygen are released if the organism is a catalase producer. The culture should not be more than 24 hours old. The required reagent is hydrogen peroxide (3% H2O2, 10 volume solution)
2H2O2. ----------------------------------------->+ 2H2O +O2
Procedure: Two mililitres (2ml) of hydrogen peroxide solution was poured into a test tube. Using a sterile wooden stick or a glass rod (not nichrome wire loops), several colonies of the test organism was removed and immersed in the hydrogen peroxide solution. Immediate bubbling was looked for.
Results:
Active bubbling…………………………….Positive catalyst test
No bubbles………………………………… Negative catalyst test
Coagulase Test
This test is used to identify Staphylococcus aureus which produces the enzyme coagulase.
Principle: Coagulase causes plasma to clot by converting fibrinogen to fibrin. Two types of coagulases are produced by most strain of Staphylococcus aureus:
Free coagulase which converts fibrinogen to fibrin by activating a coagulase-reacting factor present in plasma. Free coagulase is detected by clotting in the tube test.
Bound coagulase (Clumping factor); which converts fibrinogen directly to fibrin without requiring a coagulase-reacting factor. It can be detected by clumping of bacteria cells in a rapid slide test required
Required: EDTA anticoagulant human plasma (preferably pooled and previously HIV and hepatitis tested) or/rabbit plasma. The plasma should be allowed to warm to room temperature before being used.
Procedure: A drop of distilled water was placed on each end of a clean grease-free slide. A colony of the test organism (previously checked by gram staining) was emulsified in each of the drop to make two thick suspensions. A loopful of plasma was added to one of the suspensions, mixed gently and checked for clumping of the organisms within 10 seconds. No plasma was added to the second suspension. This was used to differentiate any granular appearance of the organism from true coagulase clumping.
Results:
Clumping within 10 seconds………………... Staphylococus aureus
No clumping within 10 seconds……………… No bound coagulase
Citrate Utility Test (Cheesebrough, 2006).
Principle: It is based on the ability of an organism to utilize citrate as its source of energy. The citrate is metabolized to acetoin and CO2.
Procedure: A light suspension of the organism was emulsified in saline. Simmom's citrate agar was stab inoculated with a straight wire loop. A growth of blue colour was observed in Simmom's agar as a positive result. This meant that citrate has been utilized.
Indole Test
Testing for indole production is important in the identification of enterobacteria. Most strains of E. coli, P. vulgaris, P. rettgeri, M. morganii and Providencia species break down the amino acid tryptophan with the release of indole.
Principle: The test organism is cultured in a medium which contains tryptophan. Indole production is detected by Kovac’s reagent which contains 4(p)- dimethylamino-benzaldehyde. This reacts with the indole to produce a red coloured compound. Kovac’s reagent is recommended in preference to Ehrlich’s reagent for the detection of indole from enterobacteria.
Procedure: The test organism was inoculated in a bijou bottle containing 3 ml of sterile tryptone water and incubated at 37oC for 48 hours. 0.5 ml of Kovac’s reagent and shook gently. Within 10minutes, it was examined for red colour in surface layer.
Result
Red surface layer………………….. Positive indole test.
No red surface layer……………….. Negative indole test.
Oxidase test
The oxidase test is used in the identification of Pseudomonas, Neisseria, Vibrio, Brucella and Pasteurella species all of which produced the enzyme cytochrome oxidase.
Principle: A piece of filter paper is soaked with a few drops of oxidase reagent, a colony of the test organism is then smeared on the filter paper. Alternatively, an oxidase reagent strip can be used when the organism is oxidase producing, the phenylenediamine in the reagent will be oxidised to a deep purple colour. Acidity inhibits oxidase enzyme activity; therefore, the oxidase test must not be performed on colonies that produced fermentation on carbohydrate-containing media such as TCBS or macconkey agar. Sub inoculation on nutrient agar is required before the oxidase test can be performed. Colonies tested from a medium that contains nitrate may give unreliable oxidase test results.
Required: Freshly prepared oxidase reagent or an oxidase reagent strip. Fresh oxidase reagent is easily oxidized. When oxidized, it appears blue and must not be used.
Procedure: The strip was moistened with a drop of sterile water. Using a piece of stick or glass rod (not an oxidized wire loop) a colony of the test organism was removed and rubbed on the strip. It was checked for a red purple colour within 20 seconds.
Result
Red purple colour…………………… positive oxidase test (Cheesbrough, 2006).
Urease test
This is used in differentiating Klebsiella species and Proteus species. The test organism is cultured in a medium which contains urea and the indicator phenol red. When the strain is urease-producing, the enzyme will break down the urea (by hydrolysis) to give ammonia and carbon dioxide. With the release of ammonia, the medium becomes alkaline as shown by a change in color of the indicator to pink-red.
The test organism was inoculated in a bijou bottle containing 3ml sterile Christensen’s modified urea broth. It was incubated at 35ºC for 12 hours. A pink color indicates a positive urease test while no pink color indicates a negative urease test.
Motility Test
Procedure: A semisolid agar medium (Sulphide indole motility (SIM) medium) was prepared in a test tube. Using a straight wire loop, the motility medium was stab inoculated with the test organism. It was inoculated at 37°C for 48hours. Bacterial motility was observed directly by examination of the tubes following incubation.
Antibacterial Susceptibility Testing
The method used was well-in-agar diffusion method.
Agar well diffusion method is widely used to evaluate the antimicrobial activity of plants or microbial extracts. Similarly to the procedure used in disk-diffusion method, the agar plate surface is inoculated by spreading a volume of the microbial inoculum over the entire agar surface. Then, a hole with a diameter of 6 to 8 mm is punched aseptically with a sterile cork borer and a volume (20–100 µL) of the antimicrobial agent or extract solution at desired concentration is introduced into the well. Then, agar plates are incubated under suitable conditions depending upon the test microorganism. The antimicrobial agent diffuses in the agar medium and inhibits the growth of the microbial strain tested
Data Analysis
Data obtained from this study was analyzed statistically using the statistical package for social sciences (SPSS) version 20 for window 8.1. The results were expressed in frequencies of occurrence and percentages.
Result
Table 4.1 shows the qualitative analysis of M. myristica and T.tetraptera using hot water and ethanol as extraction solvents. Result showed the presence of Alkaloids and phenols in moderate amounts, followed by Tanins and flavonoids in minute amounts, while Glycosides, Saponins and terpenoids where not present in the hot aqueous extract of M.myristica. Alkaloids, phenols were present in high amounts, followed by Tannins and terpenoids in moderate amounts, while Glycosides and flavonoids were in minute amounts in the ethanol extract of M.myristica Qualitative analyses of the hot aqueous extract of T.tetraptera showed that Phenols were present in high amounts followed by Alkaloids in moderate amounts, while tanins and flavonoids were present in minute amounts, but in the ethanol extracts, Alkaloids and Phenols were present in high amounts, then flavonoids in moderate amounts followed by Tanins and Terpenoids in minute amounts.
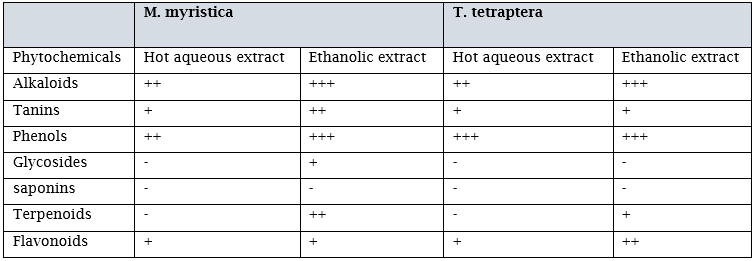
Key :+ present in minute amount, ++ present in moderate amount, +++ present in high amounts - Negative
Table 4.3 shows the antimicrobial properties of the ethanolic and hot water extract of T. tetraptera used for the study. Result showed that the ethanol extracts were more active against the gram positive and gram negative organism at all concentrations, while the hot water extract was most effective at the 10, 7.5 and 5.0 mg/ml concentrations. However when these results were compared to the control, it was observed that ciprofloxacin had higher zones of inhibition than the seed extracts.
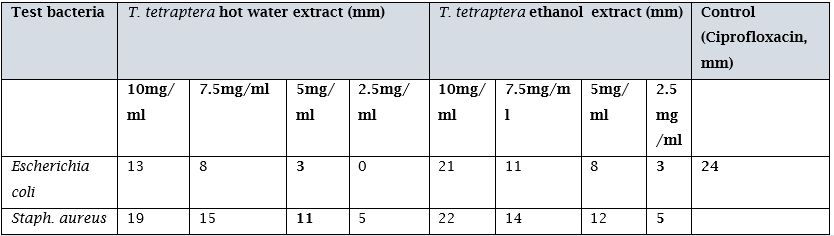
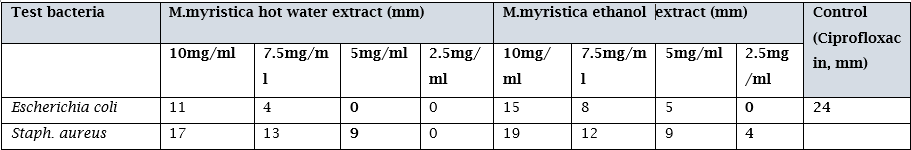
Table 4.4 shows the antimicrobial properties of the ethanolic and hot water extract of M. myristica used for the study. Result showed that the ethanol extracts were again more active against the gram negative organism at all concentrations, while the hot water extract was most effective at the 10, 7.5 and 5.0 mg/ml concentrations for both the gram negative and gram positive microorganisms. However when these results were compared to the control, it was observed that ciprofloxacin had higher zones of inhibition than the seed extracts.
Discussion
The emergence of multiple drug-resistant microorganisms is presently a global problem and a major cause of failure of the treatment of diseases [11]. Phytochemicals carry out essential medicinal roles in the body. The presence of alkaloids, phenols, saponins, steroids and tannins in Monodora myristica seeds has been reported by [12]. Also, high concentrations of phenols and phlobatannins with trace amount of tannins in ethanolic extract of Monodora myristica and Tetrapleura teraptera seeds might have accounted for its antimicrobial properties [13], these reports are in agreement with our findings. The site(s) and number of hydroxyl groups on the phenol group are thought to be related to their relative toxicity to microorganisms, with evidence that increased hydroxylation results in increased toxicity. In addition, some authors have found that more highly oxidized phenols are inhibitors. The mechanisms thought to be responsible for phenolic toxicity to microorganisms include enzyme inhibition by the oxidized compounds, possibly through more non-specific interactions with the proteins.
Almost all the metabolites detected have been suspected to contribute to antimicrobial activity of extracts in other reports [14,15,16,17]. For example, tannins have been found to form irreversible complexes with proline–rich proteins resulting in the inhibition of the cell protein synthesis; besides, herbs that contain tannin are astringent in nature and are used for treating intestinal disorders such as diarrhea and dysentery [18]. The antibacterial properties of the active plants may be due to the presence of different bioactive chemical agents in the extracts, which are known to act by a different mechanism, to exert antibacterial action. In the present study, the plant seed extracts which contained Phenols and Tanins showed to have contributed to their antibacterial activity. Mode of action of tannins may be related to their ability to inactivate several enzymes, microbial adhesion, and cell envelope transport proteins. Flavonoids and saponins have been reported to possess antibacterial activity, which could be attributed to their ability to form a complex with extracellular proteins, soluble proteins, and bacterial cell wall [19,20,21]
Antibiotic resistance is a problem that continues to challenge the healthcare sector in a large part of the world in both developing and developed countries. The emergence and spread of multidrug resistant pathogens have substantially threatened the current antibacterial therapy [22]. This has necessitated a search for a new source of antimicrobial substances such as plants as they produce a variety of bioactive compounds of known therapeutic properties. The administering of these plant seed are currently being evaluated as it is known to be part of a herbal mixtures given to women after birth for cleansing of remains of unhealthy deposits during child birth. The in-vitro studies of these plant seeds have shown significant relevance on S.aureus and E.coli at higher quantities. It was observed that T. tetraptera had a high antimicrobial activity than Monodora myristica seed extracts. Ciprofloxacin which is a conventional drug that served as our control however proved to be most effective against Staphylococcus aureus and Escherichia coli than the ethanol and aqueous extracts of the two seeds and this was also in agreement with the work of [23] who had high susceptibility to the test organisms using ciprofloxacin.
T. tetraptera pod is edible and it is commonly used in cooking as spices in several local cuisines and its medicinal properties [24]. The extracts both aqueous and ethanol showed antimicrobial susceptibility at the highest concentration of 100 mg/ml. The antimicrobial activity reported here is in line with a previous study by [25] who reported zones that ranged from 12.00 to 21.30 mm against E. coli isolates.
Inhibitory zone diameter (IZD) is measured in millimeter (mm) when disc diffusion or agar well diffusion assays is used [26]. It has been reported that plant extracts exhibiting IZD of 6mm and above against a selected pathogen are considered to possess some antimicrobial activity while suggested IZD of 10 mm and above [27]. Because many organisms are now exhibiting high resistance to most antimicrobials, this study proposes that plant extract exhibiting IZD greater than or equal to 10 mm against selected organisms should be considered to possess antimicrobial activity. From our study, it was observed that 10mg/ml and 7.5mg/ml had the highest zones of inhibition, especially with the ethanol extracts and these findings correspond with the work of [28]. The ethanolic extract has the most significant level of phytochemical components beween the two solvents used for the extraction of Monodora myristica and Tetrapleura tetraptera seeds, this depict that ethanol has the capacity to extract more phytochemical components than other solvents used in this study. The presence of these phytochemicals components in the seeds of Monodora myristica, confer it for its medicinal value
Varied susceptibility of each test organisms to the extracts usually reflect the differences in physiology of individual bacterial species as stated by [29] or differences in the quantity and quality of the active ingredients, extraction methods employed, the dosage of extract applied and the diffusion properties of these extracts in the agar [30,31]
Conclusion
The evaluation of the ethanol and aqueous extracts of M. myristica and T.tetraptera has shown to contain important bioactive compounds that can be further studied and used to produce new line of drugs that can be effective and help reduce the issues of antimicrobial resistance . It has also shown that the higher the quantity of these seed extracts, the stronger the effect it has against microorganisms. Hence, further studies can be conducted to find out more useful compounds in these seeds that can help produce safe drugs in the field of medicine.
References
-
Cheng, Y., Zhang, Y., Shi, W., Zhou, L., and Zhang, L. (2021). Development of Novel Penicillin Derivatives with Enhanced Antibacterial Activity Against Resistant Strains. Journal of Antibiotics, 74(6), 385–398.
View at Publisher | View at Google Scholar -
Garcia, J. V., Miller, A. D., and Serhan, C. N. (2021). Emerging antiviral agents: Targeting inflammation. Nature Reviews Drug Discovery, 20(5), 337–358.
View at Publisher | View at Google Scholar -
Chopra, I., and Roberts, M. (2021). Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiology and Molecular Biology Reviews, 65(2), 232–260
View at Publisher | View at Google Scholar -
Alm, R. A., and Lahiri, S. D. (2020). Narrow-Spectrum Antibacterial Agents—Benefits and Challenges. Antibiotics, 9(7), 418.
View at Publisher | View at Google Scholar -
Podolsky SH. (2018). The Evolving Response to Antibiotic Resistance (1945-2018). Palgrave Commun. 4, 124.
View at Publisher | View at Google Scholar -
Rallis, D., Giapros, V., Serbis, A., Kosmeri, C., and Baltogianni, M. (2023). Fighting Antimicrobial Resistance in Neonatal Intensive Care Units: Rational Use of Antibiotics in Neonatal Sepsis. Antibiotics, 12(3), 508.
View at Publisher | View at Google Scholar -
7.Anderson, J. R. (2020). Disinfection, sterilization, and antisepsis: An overview. In Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (9).
View at Publisher | View at Google Scholar -
Barker, A. K., and Jones, C. L. (2018). Antiseptics. In Stat Pearls. StatPearls Publishing.
View at Publisher | View at Google Scholar -
Vakulenko, S. B., and Mobashery, S. (2020). Versatility of Aminoglycosides and Prospects for Their Future. Clinical Microbiology Reviews, 33(4), e00068-19.
View at Publisher | View at Google Scholar -
White, T. C., and Johnson, E. M. (2016). The challenge of treating fungal infections: Highlighting the need for new antifungal drugs. Discovery Medicine, 22(122), 277–280.
View at Publisher | View at Google Scholar -
Brown, E. D., and Wright, G. D. (2016). Antibacterial drug discovery in the resistance era. Nature, 529(7586), 336–343.
View at Publisher | View at Google Scholar -
McGuinness, W. A., Malachowa, N., and DeLeo, F. R. (2017). Vancomycin resistance in Staphylococcus aureus. Yale Journal of Biology and Medicine, 90(2), 269–281
View at Publisher | View at Google Scholar -
Olayode, O. O. (2020). Germination Investigations of Monodoramyristica (Gaertn.) Dunal Progenies. Journal of Ecology and Natural Resources, 4(4). https://doi.org/10.23880/jenr-16000206
View at Publisher | View at Google Scholar -
Germovsek, E., Barker, C. I., and Sharland, M. (2016). What do I need to know about aminoglycoside antibiotics? Archives of Disease in Childhood - Education and Practice Edition, 102(2), 89–93.
View at Publisher | View at Google Scholar -
Heinze, G., andSteinhagen, F. (2020). Folate antagonists. Pharmacotherapy, 38(5), 576–592.
View at Publisher | View at Google Scholar -
World Health Organization. (2021). Antimicrobial Resistance. Retrieved from https://www.who.int/news-room/q-a-detail/antimicrobial-resistance
View at Publisher | View at Google Scholar -
Barker, C. I., Germovsek, E., and Sharland, M. (2016). What do I need to know about penicillin antibiotics? Archives of Disease in Childhood - Education and Practice Edition, 102(1), 44–50.
View at Publisher | View at Google Scholar -
Jimoh, A.T. Ajao, R.F. Zakariyah, M.B. Odebisi-Omokanye,and H. O. Abdulrahman A. (2020).Nigeria Journal Pure and Applied Science33(1): 3674-3686.
View at Publisher | View at Google Scholar -
Kudi AC, Umoh JU, Eduvie LO,andGefuJ.(1999). Screening of some Nigerian medicinal plants for antibacterial activity. Journal of Ethnopharmacology. 67:225–8.
View at Publisher | View at Google Scholar -
Leclercq, R., andCourvalin, P. (2021). Resistance to glycopeptides in enterococci. Clinical Infectious Diseases, 13(Supplement_7), S339–S346.
View at Publisher | View at Google Scholar -
Cowan, M. M. (2023).
View at Publisher | View at Google Scholar -
Ebana, R. U. B., Edet, U., Ekanemesang, U., Ikon, G., Etok, C., and Edet, A. (2016). Antimicrobial activity, phytochemical screening and nutrient analysis of Tetrapleura tetraptera and Piper guineense. Asian Journal of medicine and Health, 1(3), 1-8.
View at Publisher | View at Google Scholar -
Bush, K., and Bradford, P. A. (2019). Interplay between β-lactamases and new β-lactamase inhibitors. Nature Reviews Microbiology, 17(5), 295–306.
View at Publisher | View at Google Scholar -
Campbell, E. A., Korzheva, N., Mustaev, A., Murakami, K., Nair, S., Goldfarb, A., and Darst, S. A. (2018). Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell, 104(6), 901–912.
View at Publisher | View at Google Scholar -
Falagas, M. E., Kasiakou, S. K., andSaravolatz, L. D. (2015). Colistin: The revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clinical Infectious Diseases, 40(9), 1333–1341.
View at Publisher | View at Google Scholar -
Lee, E. J., Park, J., Lee, G., and Kim, D. S. (2019). The Use of Broad-spectrum Antibiotics and Antibiotics to Treat Antimicrobial-Resistant Bacteria. YakhakHoeji, 63(1), 43–53.
View at Publisher | View at Google Scholar -
Brown, E. D., and Jones, R. N. (2019). Carbapenems: A new chapter in the β-lactam tale. The Lancet Infectious Diseases, 19(3), 238–240
View at Publisher | View at Google Scholar -
Manita, M., and Kumar Nepal, T. (2021). An Updated Checklist of Globally Threatened Species in
View at Publisher | View at Google Scholar -
Bhutan as Listed in IUCN Red List of Threatened Species. International Journal of Science and Research (IJSR), 10(2), 1640–1646.
View at Publisher | View at Google Scholar -
Outterson K, (2019). Developing new antibiotics: The who, what, and how of antimicrobial innovation. Clinical Microbiology Infections. 25(12), 1477-1481.
View at Publisher | View at Google Scholar -
Becker, K., Brune, W., and Kohlmann, T. (2019). Macrolide Resistance in Streptococcus pneumoniae Isolates from Patients with Invasive and Non-Invasive Infections. Infection, 33(3), 122–125.
View at Publisher | View at Google Scholar -
Robinson, T., and Dalton, J. P. (2017). Zoonotic helminth infections with particular emphasis on fasciolosis and other trematodiases. Philosophical Transactions of the Royal Society B: Biological Sciences, 372(1712), 20160057.
View at Publisher | View at Google Scholar






