Research Article | DOI: https://doi.org/WHRWB/RA/002.
Antibacterial Activities Of Some Of Medicated Soaps (Dettol,Tetmosol,Tura) on Staphylococcus Aureus Isolated From Wound Infection In Rhema Unversity Teaching Hospital (Ruth)
Abstract
Initial signs of wound infection may include bacterial colonization; genuine infection only happens when colonization is pairedwith additional elementslike reduced vascularsupply, intrinsic pathogenicity of particular bacteria, and host immunological factors. The goal of thisexperiment was to ascertain whether three differentvarieties of medicated soap could inhibit the growth of Staphylococcus aureusthat was isolated from a wound infection. Twenty samples in all, from patients with wound infections between the ages of nine and forty-nine, were taken at Rhema University Teaching Hospital. Using sterile swab sticks, twenty (20) wound samples from patients getting care at Rhema University Teaching Hospital were aseptically sampled. The test organism, Staphylococcus aureus, was isolated after it was inoculated and subcultured. Standard microbiological techniques were used to identify the isolate. All three soaps' antibacterial properties against particular bacterial strains were assessed in millimeters and documented as inhibition zones. The Staphylococcus aureus inhibition zone values with these soaps (Dettol, Tetmosol, and Tura). The work's inhibition zone values demonstrated how these antibacterial soaps differed from one another in their mode of action. The findings indicate that when the concentration of these soaps (Dettol, Tetmosol, and Tura) was raised, so did their antibacterial properties. It implies that the stronger antibacterial activity of the more concentrated solution. The results showed that the zone of inhibition for Tura soap was 24.00 mm, the highest for Tetmosol (14.00mm), and the lowest for Dettol (12.00 mm). The outcome demonstrated the effectiveness of medicated soaps against the staphylococcus aureus responsible for human wound infections. Therefore, the usage of medicated soap can help stop the spread of skin and wound pathogens as well as skin infections.Additionally, since extendeduse destroys both harmful bacteria and healthy flora, it is advised against it.
Introduction:
Since the skin is the body's first line of defense, the majority of bacteria, including Staphylococcus aureus (S. aureus) and Pseudomonas aureginosa, dwell there and are the main cause of skin infections. Staphylococcus aureus is a human pathogen in addition to a commensal bacterium. About thirty percent of people are infected with Staphylococcus aureus [1]. A fracture-induced break in the skin's continuity or integrity is called a wound. readily become infected on the skin's surface due to a wide range of organisms colonizing there. Several distinct types of bacteria have been identified in some research, isolated from patients residing in various geographic locations. Inflammatory microorganisms belong to the class of pyogenic bacteria [2].
Pyoderma moderate to severe necrotizing infections are examples of skin infections. A wide range of microorganisms, such as fungi, bacteria, and parasites, can infect skin. Bacteria are the most common cause of skin infections. The most frequent gram-positive bacteria that cause skin infections are Staphylococcus aureus and hemolytic streptococcus. A multipurpose pathogen, Staphylococcus aureus can infect people with a wide range of diseases, including infections and soft tissue infections. Staphylococcus aureus infections of the skin and soft tissues are serious hospital-acquired and community-acquired infections that pose a serious risk to health [3]. Antiseptic soap, which contains phenolic compounds that help keep organisms like S. aureus away from the sites, can help wash the wound surface and other infected skin lesions, such as atopic dermatitis, in order to facilitate the biological process of wound healing, which involves regeneration, cell proliferation, and collage production. [4]In addition to their ability to reduce pyogenic skin infections caused by Staphylococcus aureus and other gram-negative bacteria, active components in soaps also have antibacterial action. For many years, soaps and other cleaning agents have been used extensively for a variety of cleaning tasks.Soap that has chemical compounds supposedly helping to destroy bacteria is called antibacterial soap.Both cleaning and eliminating microbes need the use of soaps[5].
Soap is enhanced with certain active ingredients to strengthen its antimicrobial capabilities. Though other chemical additions are also frequently used, triclosan is the main ingredient in most antibacterial soaps [6]. In soaps, triclocarban and triclosan are the most often utilized antibacterial ingredients. But soaps also frequently contain benzalkonium chloride, benzethonium chloride, and chloroxylenol as antibacterial components [7].
The bacterial flora on human skin can be eliminated by 65–85% with antibacterial soap. Antibacterial activity is the ability to eradicate or hinder the growth of microorganisms. These are known as static or cidal effects, depending on the circumstances. This is essential to prevent skin infections and sepsis in people [8].
Antibiotic-resistant microorganisms could be produced by antibacterial soaps. Overuse of antibiotics can lead to resistance, which is the product of a small percentage of the bacterial population undergoing a random mutation that makes it resistant to the drug. Other bacteria will be killed by that chemical if it is applied often enough, but this resistant group will be able to multiply. This can effectively render that chemical ineffective against the bacterial strain if it occurs widely enough [10].
In the field of medicine, this is currently a major issue that the WHO refers to as a "threat to global health security." Certain bacterial species, like MRSA, have developed resistance to many medications, making it more difficult to manage and treat infections as they spread. Health experts assert that more investigation is necessary before concluding that triclosan is contributing to resistance; however, a number of studies have raised the possibility [11].
Infections from bacteria on wounds are a public health concern. Since the skin is the body's first line of defense, most bacteria, including Staphylococcus aureus, live there and are mostly responsible for wound infections when the skin's integrity is weakened. Research on the antibacterial activity of the medicated soaps (Dettol, Tetmosl, and Tura) on Staphylococus aureus isolated from wound infection is necessary because of the issue of antibiotic resistance, which has made the prospect of using medicated soap as an alternative medication very popular. However, it is still necessary to use the most effective and selective medication available [12].
When wound infections are not adequately treated, they can result in consequences like delayed healing, scarring, and in the worst instance, amputation. Since Staphylococcus aureus is the most common bacteria isolated from wounds, washing the wound's surface with antibacterial soap prior to applying more medication is essential to preventing the growth of bacteria.
Study Design
This study involved the evaluation of antibacterial activity of some medicated soaps on staphylococcus aureus isolated from infected wound swab.
Study Area
Samples used in this study were collected from Rhema University Teaching Hospital Abia State. Patients who were receiving treatment for wound infections at the surgical ward and emergency unit of Rhema University Teaching Hospital were included in the study. Laboratory analysis was carried out in the Medical Microbiology laboratory department of Medical Laboratory Science , Rhema University, Aba,Abia State.
Ethical Clearance
Ethical approval to carry out this work in Rhema University was sought and obtained from the Ethics and Research Committee of the College of Medicine and health Sciences of Rhema University, Abia State.
Inclusion Criteria
Patients with acute and chronic wounds were included in the study and those who gave their consent.
Exclusion Criteria
Patients without acute or chronic wounds as well as those who did not give consent to take part in the research were excluded from the study.
Specimen Collection and Transport
Wound swabs were collected by gentle cleansing of a skin wound prior to sample collection is recommended to reduce commensal flora contamination. After superficial precleansing of wounds with physiologic saline, each specimen was collected by rotating a sterile, premoistened swab (NuovaAptaca SRL, Canelli, Italy) across the wound surface of a 1 cm2 area in a zig-zag motion, from the centre to the outside of the wound. Purulent exudates were expressed onto swabs. Wound swabs were placed into the transport media, Labelled with patient’s full name, date of birth or health card number, source of specimen and date and time of collection. Swabs were maintained at room temperature and submitted to the laboratory within 24 hours of collection for further analysis.
Culture And Identification Sample processing
The swabs were subjected to microbiological techniques for the isolation, identification and antibiotic susceptibility testing in the laboratory.
Isolation method
All specimens collected were immediately inoculated on freshly prepared blood agar, MacConkey agar and Nutrient agar, and incubated at 37°C for 24 hrs. After incubation, the bacterial colonies were observed and discrete colonies were picked and purified by sub-culturing onto freshly prepared Nutrient agar, MacConkey agar and blood agar using a streak plate technique. Isolated colonies that grew on the plates were then transferred onto Nutrient agar slants with a proper label. These agar slants were stored in the refrigerator at 4°C and were used for further characterization Conventional Methods of Characterization and Identification Of Isolates
The following tests were carried out for the characterization and identification of the bacteria: Gram staining, Catalase and Coagulase test.
Preparation Of the Soap Solution
With the aid of a weighing balance, 1g of each medicated soap (Dettol, Tetmosol, Tura) was weighed and cut into bits using sterile surgical blade and poured into 3 labeled sterile conical flask. 10ml of distilled water was used to emulsify the soap sample in each conical flask respectively.
Antimicrobial testing of the medicated soap solution against the staphylococcus aureus isolates:
The antimicrobial activity was evaluated using the agar well diffusion method. Prior to use, of the test organisms were sub- cultured on nutrient broth and incubated at 37 degrees Centigrade for 24 hours. The concentration of the 24 hours culture was adjusted to 0.5 McFarland Standard. 0.1 ml volume of the standard suspension of each test bacterial strain was spread evenly on Muller Hinton agar plates using sterile L-shaped glass rod and the plates were allowed to dry at room temperature. Subsequently, 6 mm-diameter wells were bored on the agar and100 μl of each reconstituted soap solution was dispensed into triplicate wells. The plates were allowed to stand on flat surface at room temperature for 1 hour to allow proper diffusion of soap solution into the agar, the inoculated plates were then incubated at 37 o C for 24 h and the inhibition zone diameter (IZD) were measured in millimeters (mm). Antimicrobial activities were expressed as the IZD (mm) produced by the medicated soaps.
Result:
Table 1 shows the identification of staphylococcus aureus isolated for the study. This was done using their cultural andbiochemical characteristics, macroscopically and microscopically. Culturalcharacteristics show that Staphylococcus aureus appeared golden yellow on nutrient agar plates and yellow on mannitol salt agar plates. It was also observed microscopically that it is gram positive and arranged in clusters. Biochemical test also showed that the isolateswere catalase and coagulase positive.
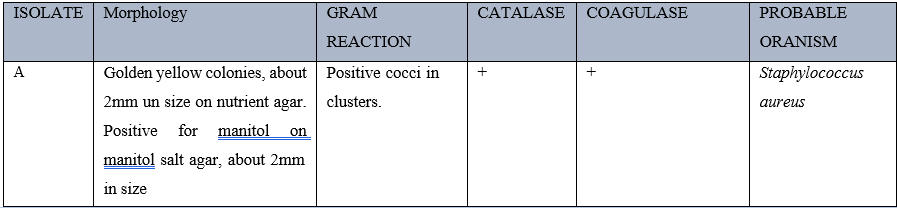
Table 2 shows the wound sites of people attending Rhema university teaching hospital according to gender. It was observed that most of the wound patients were males and people who had accident wounds were more (30%), followed by individuals with Leg Ulcers (20%) while wound cases from fire burns were the least (10%).
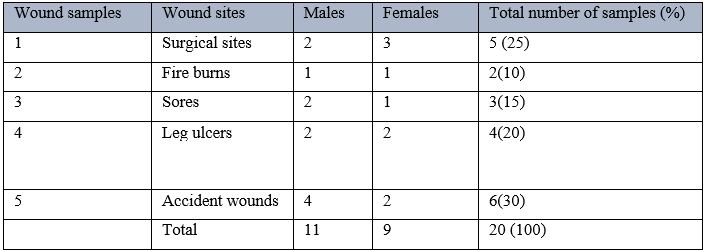
Key: number in parenthesis represent the percentage of the individuals with various wounds
Table 3 shows the occurrence of Staphylococcus aureus from the wound samples and result show that Staphylococcus aureus occurred mostly in surgical sites and sores, with a percentage of 40. However, Staphylococcus aureus did not occur on fire buns and accident wounds.
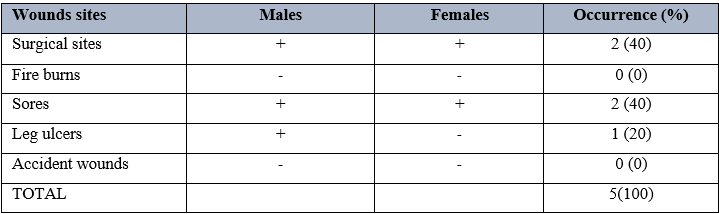
Table 4 shows the antimicrobial activity of Dettol soap on Staphylococcus aureus using different grams. It was observed that 1.0grams had the highest zones of inhibition of 12mm, followed by 0.75 grams with a zone of inhibition of 7mm. there was however no zone of inhibition observed when 0.125gran concentration was used. When this was compared to the control, result showed that Dettol soap was effective
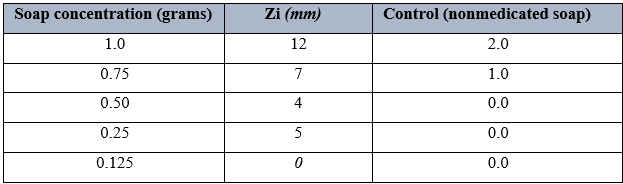
Table 5 shows the antimicrobial activity of Tura soap on Staphylococcus aureus using different grams. It was observed that 1.0grams had the highest zones of inhibition of 24mm, followed by 0.75 grams with a zone of inhibition of 19mm. there was however no zone of inhibition observed when 0.125gran concentration was used. When this was compared to the control, result showed that Tura soap was effective.
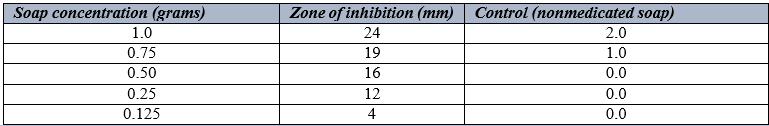
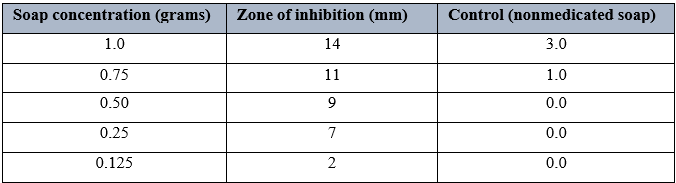
Table.6 shows the antimicrobial activity of Tetmosol soap on Staphylococcus aureus using different grams. It was observed that 1.0grams had the highest zones of inhibition of 14mm, followed by 0.75 grams with a zone of inhibition of 11mm. there was however the least zone of inhibition was observed with 0.125grams concentration, having a zone of inhibition of 2mm. When this was compared to the control, result showed that Tura soap was effective.
Key: ZI- Zone of inhibition; MM- millimeters
Discussion
This study examined the antibacterial properties of medicated soaps (Tetmosol, Dettol, and Tura) against Staphylococcus aureus isolated from wound infection sites. According to the report, the majority of patients receiving care at Living Word Hospital had wounds from surgeries (25%) fire burns (10%), sores (15%), leg ulcers (20%), and accidents (30%). Additionally, it was noted that there were more men than women with injuries. This could be because the majority of accident victims in emergency rooms are typically motorcyclists, keke drivers, bus drivers, etc. Animal and/or vegetable fats are mixed with caustic soda (sodium hydroxide) or caustic potash (potassium hydroxide) in a procedure known as saponification (oils) to produce soap, a chemical that is soluble in water. Soap can be made as bars, granules, or tablets for use as a cleaning agent, just like other antiseptic soaps [13]. The study's findings demonstrated that, while to varying degrees, the medicinal soaps under test had antibacterial activity, as shown by the inhibition of the isolates' growth patterns. Since the activities of the soap extracts were unaffected by the Gram reaction, their range of activity was likewise broad. Soaps are used to clean and eliminate dust and bacteria from a range of surfaces, such as skin, clothing, and cutlery [14].
Similar research was done on Staphylococcus aureus that was isolated from wound infections to test the antibacterial activity of a few chosen medicated soaps, including Dettol, Tura, Sanitol, Safeguard, and Tetmosol. As a result, their findings demonstrated that as the concentration of these soaps (Tura, Dettol, Sanittol, Safeguard, and Tetmosol) grew, so did their antibacterial activity [15]. Additionally, a study was conducted to evaluate the antibacterial activity of several antiseptic soap types on S. aureus isolated from eczematous lesions and wound infections [16]. However, since the organisms examined shown sensitivity to the majority of these soaps at greater concentrations, these results were consistent with the findings of the current study. Generally speaking, soaps are used to clean and to get rid of dust and bacteria that are on the skin's surface [17]. Individuals have different preferences when it comes to soap, but it should be gentle on delicate skin and capable of eliminating harmful bacteria from the skin. All three soaps' antibacterial properties against particular bacterial strains were assessed in millimeters and documented as inhibition zones.Table 4- 6 above displays the Staphylococcus aureus inhibition zone values when using these soap brands.These antibacterial soaps differed from one another in their modes of action, as seen by the inhibition zone values presented in Figure 1. The findings indicate that when the concentration of these soaps—Tura, Dettol, and Tetmosol—was raised, so were their antibacterial properties. It implies that the stronger antibacterial activity of the more concentrated solution. As per the obtained results, the zone of inhibition for Tura soap is the highest at 24.00mm, followed by that of Tetmosol at 14.00mm, and Dettol at 12.00mm. My results were consistent with those of [18], who conducted research on comparable samples in Wukari, Taba state. The observed results also demonstrated that the different medicated soaps were more effective in killing the test organism at higher concentrations.
According to their contents, the soaps used in this investigation contained triclosan and triclocarban, which are potent antibacterial agents. These results align with those of [19], who also detected these compounds during the course of their investigation. As the experiment's control, the Beauty soap sample showed no appreciable inhibition against the test organism. This explains why beauty soaps are used as regular soaps, mainly to remove dirt from body surfaces and leave perfume on the skin, rather than being recommended as drugs to control infections [20].
Conclusion
According to this study, washing the area around wound surfaces with medicated soaps helps lower the concentration of Staphylococcus aureus. It's also crucial to remember that a soap's activity is mostly dependent on how much is used at any given time. This study has also shown how effective these soaps are as antibacterial agents, proving that they should be used to cleanse people with any kind of skin wound.
References
-
Nwankwo, I. U., Edwards, K. C., Itaman, V. O., Udensi, C. G., & Unah, O. G. (2022). Antibacterial Activities of Medicated and Antiseptic Soaps on Staphylococcus Aureus and Pseudomonas Aeruginosa Isolated from Wound Infection. Antibacterial Activities of Medicated and Antiseptic Soaps on Staphylococcus Aureus and Pseudomonas Aeruginosa Isolated from Wound Infection, 8(83), 39–45.
View at Publisher | View at Google Scholar -
Frykberg, R. G., & Banks, J. (2015). Challenges in the Treatment of Chronic Wounds. Advances in Wound Care, 4(9), 560–582.
View at Publisher | View at Google Scholar -
Ike, C.C. (2016). Antibacterial activities of different antiseptic soaps sold in Aba on Staphylococcus aureus from clinical samples. Journal of Biological Science. 2(7).
View at Publisher | View at Google Scholar -
Chaudhari, V.M. (2016). Studies on antimicrobial activity of antiseptic soaps and herbal soaps against selected human pathogens. Journal of Scientific Innovative Research, 5(6):201- 204.
View at Publisher | View at Google Scholar -
Almuhayawi, M. S., Alruhaili, M. H., Gattan, H. S., Mohanned Talal Alharbi, Nagshabandi, M. K., Soad Al Jaouni, Selim, S., Awadh Alanazi, Yasir Alruwaili, Osama Ahmed Faried, & Elnosary, M. E. (2023). Staphylococcus aureus Induced Wound Infections Which Antimicrobial Resistance, Methicillin- and Vancomycin-Resistant: Assessment of Emergence and Cross Sectional Study. Infection and Drug Resistance, Volume 16(2), 5335–5346.
View at Publisher | View at Google Scholar -
Nunan, R., Harding, K. G., & Martin, P. (2014). Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity. Disease Models & Mechanisms, 7(11), 1205–1213.
View at Publisher | View at Google Scholar -
Ekawati, E. R., Darmanto, W., & Wahyuningsih, S. P. A. (2020). Detection of Staphylococcus aureus in wound infection on the skin surface. IOP Conference Series: Earth and Environmental Science, 456(1), 012-038.
View at Publisher | View at Google Scholar -
Imarenezor, E. P. K., Ebuara, F. U., Abhadionmhen, O. A., Brown, S. T. C., Apine, D., and Isaac, K., 2020.
View at Publisher | View at Google Scholar -
Mihai, M. M., Preda, M., Lungu, I., Gestal, M. C., Popa, M. I., & Holban, A. M. (2018). Nanocoatings for Chronic Wound Repair—Modulation of Microbial Colonization and Biofilm Formation. International Journal of Molecular Sciences, 19(4), 11-79.
View at Publisher | View at Google Scholar -
Sen, C. K. (2021). Human Wounds and its Burden: Updated 2020 Compendium of Estimates. Advances in Wound Care,10(5).
View at Publisher | View at Google Scholar -
Wu, S., Applewhite, A. J., Niezgoda, J., Snyder, R., Shah, J., Cullen, B., Schultz, G., Harrison, J., Hill, R., Howell, M., Speyrer, M., Utra, H., de Leon, J., Lee, W., & Treadwell, T. (2017). Oxidized Regenerated Cellulose/Collagen Dressings: Review of Evidence and Recommendations. Advances in Skin & Wound Care, 30(11), 1–18.
View at Publisher | View at Google Scholar -
Mustoe, T. (2004). Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy. The American Journal of Surgery, 187(5), 65–70.
View at Publisher | View at Google Scholar -
Richard, J.-L., Lavigne, J.-P., & Sotto, A. (2012). Diabetes and foot infection: more than double trouble. Diabetes/Metabolism Research and Reviews, 28(1), 46–53
View at Publisher | View at Google Scholar -
Jneid, J., Lavigne, J. P., La Scola, B., & Cassir, N. (2017). The diabetic foot microbiota: A review. Human Microbiome Journal, 5-6(1), 1–6.
View at Publisher | View at Google Scholar -
Malone, M., Bjarnsholt, T., McBain, A. J., James, G. A., Stoodley, P., Leaper, D., Tachi, M., Schultz, G., Swanson, T., & Wolcott, R. D. (2017). The prevalence of biofilms in chronic wounds: a systematic review and meta-analysis of published data. Journal of Wound Care, 26(1), 20–25.
View at Publisher | View at Google Scholar -
Sisay, M., Worku, T., & Edessa, D. (2019). Microbial epidemiology and antimicrobial resistance patterns of wound infection in Ethiopia: a meta-analysis of laboratory-based cross-sectional studies. BMC Pharmacology and Toxicology, 20(1). 7-9
View at Publisher | View at Google Scholar -
17
View at Publisher | View at Google Scholar -
Wilkins, R. G., & Unverdorben, M. (2013). Wound Cleaning and Wound Healing. Advances in Skin & Wound Care, 26(4), 160–163.
View at Publisher | View at Google Scholar -
Shettigar, K., & Murali, T. S. (2020). Virulence factors and clonal diversity of Staphylococcus aureus in colonization and wound infection with emphasis on diabetic foot infection. European Journal of Clinical Microbiology & Infectious Diseases, 39(12), 2235–2246.
View at Publisher | View at Google Scholar






