Case Report | DOI: https://doi.org/CCSRR-CR-25-025
Atypical Presentation of Left Ventricular Non-Compaction Cardiomyopathy: A Case Report
Abstract
Left Ventricular Non-Compaction (LVNC) is a rare and intriguing cardiomyopathy characterized by the failure of myocardial compaction during embryogenesis, resulting in a spongy appearance of the left ventricular myocardium. Despite its first pathological identification nearly a century ago, LVNC remains a diagnostic challenge, often presenting with overlapping features of hypertrophic and dilated cardiomyopathies. This case report details the clinical journey of a 42-year-old Comorian male presenting with acute decompensated heart failure, bilateral lower limb edema, and hemoptysis, compounded by a history of smoking, alcohol consumption, and familial sudden cardiac death. Comprehensive diagnostic evaluation confirmed LVNC. The case emphasizes the importance of advanced imaging modalities and highlights the complexities of managing LVNC, including anticoagulation, heart failure therapy, and addressing thromboembolic risks. Additionally, the coexistence of endomyocardial fibrosis (EMF), an endemic restrictive cardiomyopathy, further underscores the diagnostic and therapeutic challenges in resource-limited settings.
Abbreviations
(LVNC) - Left Ventricular Non-compaction
(RV) - Left Ventricle (LV), Right Ventricle
(TR) -Tricuspid regurgitation
(EF) - Ejection Fraction
(ESV) - End Systolic Volume
(EDV) - End Diastolic Volume
(EMF) - Endomyocardial Fibrosis
Introduction:
Left Ventricular Non-Compaction (LVNC) is a rare congenital cardiomyopathy resulting from the failure of myocardial compaction during embryogenesis. This condition is characterized by prominent trabeculations and deep intertrabecular recesses within the myocardium, which persist postnatally. Although trabecular compaction typically progresses during normal cardiac development, disruption in this process leads to LVNC. While the condition is more commonly observed in the right ventricle, its occurrence in the left ventricle is far less common [1]. Left Ventricular Non-compaction is a very rare cardiac morphology with an especially low incidence in the adult of 0.05% [2]. The first description and reporting of a spongy myocardium was by Grant 1926 [3]. It currently remains an unclassified type of cardiomyopathy, not being restrictive, dilated, or hypertrophic [4].
This case report highlights the clinical presentation, diagnostic findings and management of a 42-year-old Comorian man with a history of smoking and alcohol consumption who presented to the emergency department with acute heart failure. The patient exhibited progressive dyspnea, orthopnea and paroxysmal nocturnal dyspnea over three weeks, followed by hemoptysis, intermittent fever and lower limb edema. His significant cardiac findings included severe global left ventricular hypokinesia with a markedly reduced ejection fraction and multiple left ventricular thrombi observed on cardiac MRI. These findings were highly suggestive of LVNC raising differential diagnosis. This case underscores the diagnostic complexity and management challenges associated with LVNC, highlighting the importance of imaging modalities and the role of anticoagulation in preventing thromboembolic complications.
Case presentation:
A 42-year-old Comorian man presented to the emergency department with progressive dyspnea, orthopnea and paroxysmal nocturnal dyspnea for the past three weeks, he presented after developing cough productive of blood-tinged sputum associated with chills, and intermittent fever. He reported progressive swelling of his legs in the past three weeks.
He had been a smoker for the last 15 years, but quit smoking three weeks before presentation. His alcohol consumption was three cans of beer three times a week (21 units/ week). Family history was significant for sudden cardiac death in his father at the age of 50.
On admission his vitals were blood pressure 113/94 mmHg, heart rate 101 bpm, respiratory rate of 18 breaths per minute, SPO2 of 99% on nasal cannula 4L, and temperature of 36.7 °C.
On examination there was jugular venous distention with estimated right atrial pressure of 9-10 cmH2O, decreased air entry on the bases of both lungs bilaterally, more prominent on the right side, decreased tactile vocal fremitus, and fine crackles up to mid-lung fields.
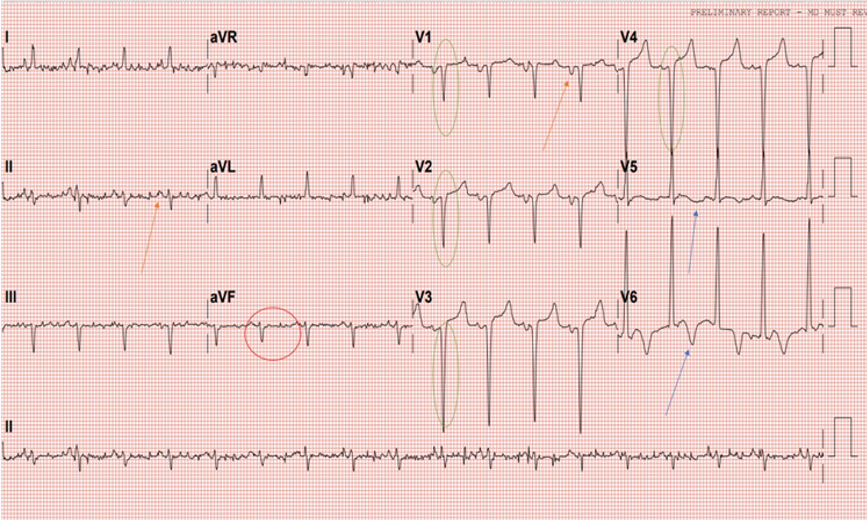
Abdomen was soft with no tenderness, with 1-2 cm liver enlargement below the right costal margin. Cardiovascular examination showed S1 S2 and S3 sounds with no murmurs. Lower limb examination displayed grade II-III bilateral lower limb edema. ECG (Figure 1) displayed sinus rhythm, left axis deviation, left atrial enlargement, pathological Qs in V1-V4 as well as T-wave inversions in V5 and V6.
Patient was admitted as a case of de novo decompensated heart failure with community acquired pneumonia, hence he was started on antibiotics according to the hospital guidelines, IV furosemide and guideline directed medical therapy for heart failure: Bisoprolol once daily (OD), spironolactone 25 mg OD, furosemide (10mg/ml), empagliflozin 10 mg od, sacubitril-valsartan 24mg, and prophylactic dose of enoxaparin.
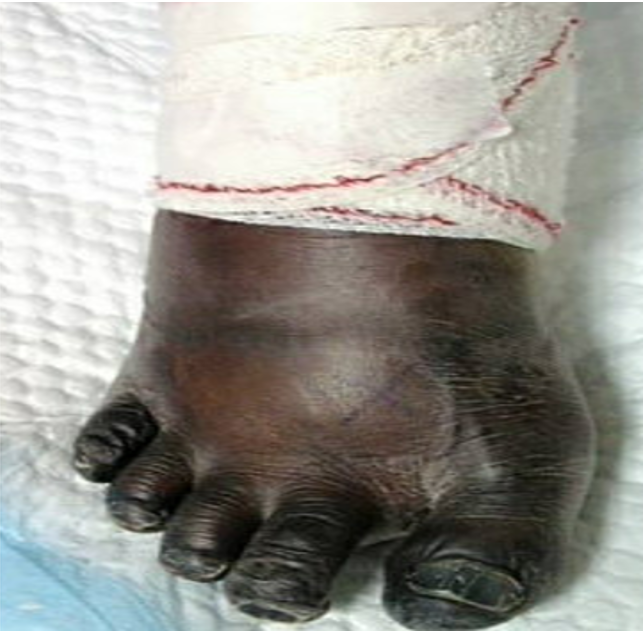
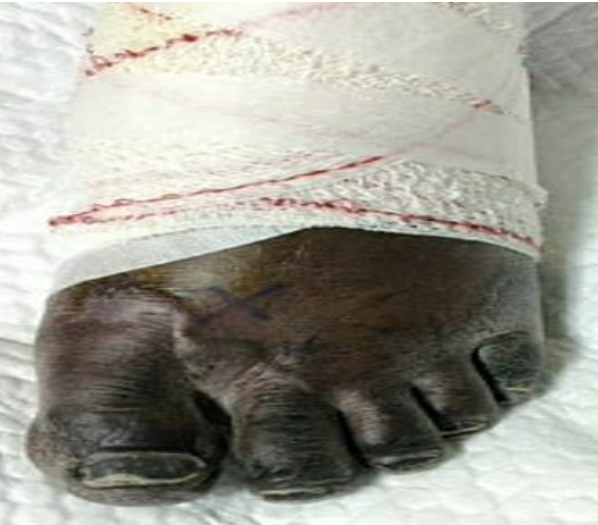
Despite improvement in signs and symptoms of acute heart failure and pneumonia, patient developed bluish discoloration of his toes bilaterally (Figure 2, 3). He was seen by the vascular team and diagnosed as a case of microembolization as pulses were present bilaterally with triphasic pulse waves on Doppler ultrasound. Therapeutic anticoagulation was initiated.
Abdominal ultrasound showed enlarged liver with mild course echogenicity, consistent with liver congestion, bilateral renal grade 3 changes and small left sided pleural effusion.
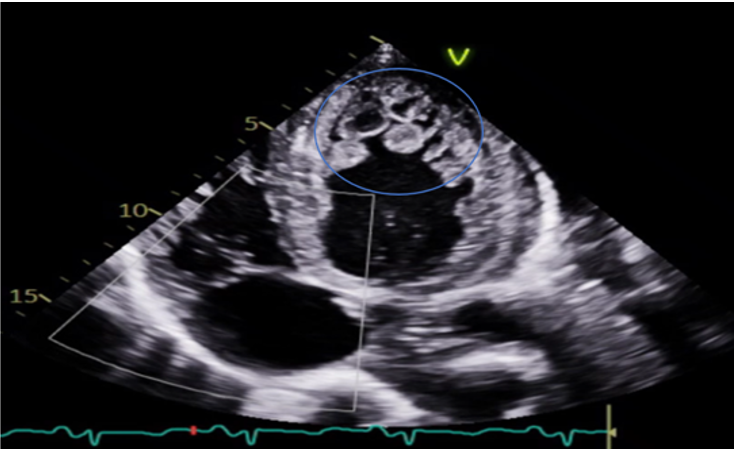
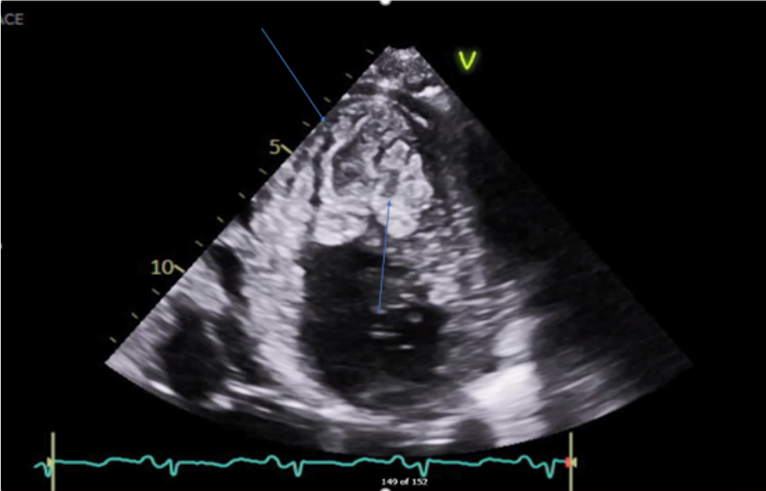
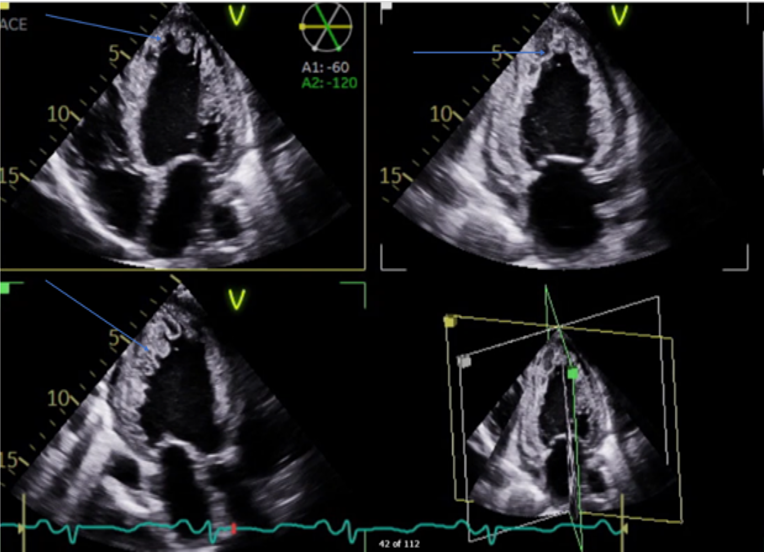
Transthoracic Echocardiography (Figure 4, 5, 6) showed normal Left ventricular (LV) size with significant left ventricular hypertrophy (LVH), ejection fraction (EF) of 10-15%, severe global LV hypokinesia, compacted and non-compacted myocardium were noticed in the left ventricle with a ratio of non-compacted/compacted of 2:1. Right ventricle was mildly dilated with depressed systolic function tricuspid annular plane systolic excursion (TAPSE) = 1.2. Moderate to severe Tricuspid regurgitation (TR) with TR jet velocity of 3.4 m/s, inferior-vena cava (IVC) = 2.3 with less than 50% collapse and mild pericardial effusion.
The final impression of LVNC is highly likely, with additional findings of global longitudinal strain of 8.4% (normal 17–24%), prominent trabeculations in LV mainly involving lateral, inferior, mid to distal septal, apex, and RV free wall suggestive of non - compaction cardiomyopathy, there is continuity of large trabeculations giving multiple cystic appearance of variable sizes with high suspicion of LV clots present in LV apex with central liquefaction.
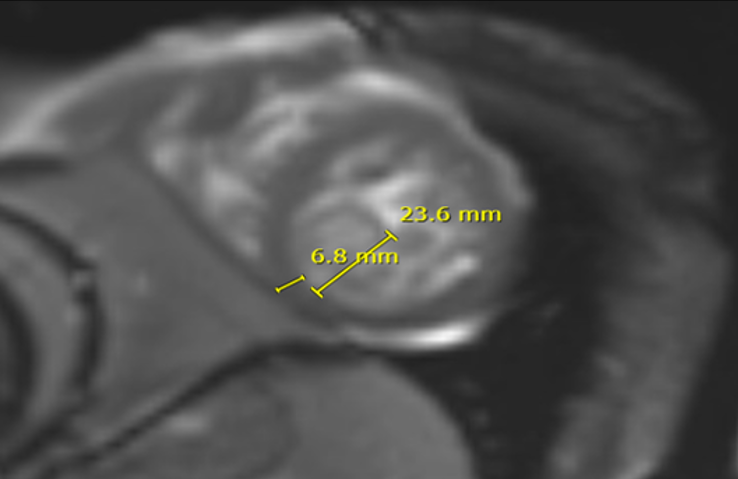
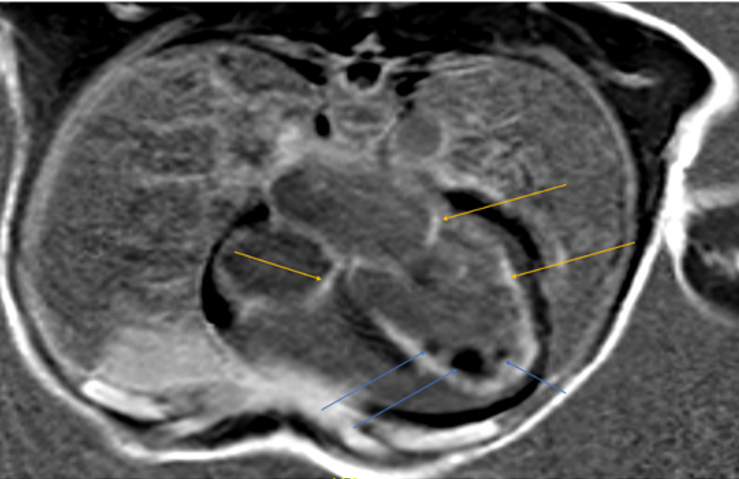
Cardiac MRI (Figure 7, 8) showed left ventricle with significant increased indexed end systolic volume (ESV) and slightly increased indexed end diastolic volume (EDV) with low EF (24%). Global hypokinesia with normal LV wall thickness was observed. There was also increased non-compacted myocardium with a non-compacted to compacted myocardium ratio of 3.7 in the apical region. Increased mass index and multiple LV Thrombi were noted (Figure 8).
Right ventricle was found to have increased indexed systolic volume, mild increased EDV with low EF (18%) and global hypokinesia. Endocardial enhancement of the LV and enhancement of both atrial walls.
T1 mapping values were as follows:
- Pre-contrast (ventricle): 1009 m/s
- Pre-contrast (blood): 1442 m/s
- Post-contrast (septum): 441 m/s
- Post-contrast (blood): 329 m/s
Hematocrit was 53, Extracellular Volume (ECV) fraction was 26% indicating no interstitial fibrosis. Bilateral lower lobes consolidation was present due to inflammatory process.
Endomyocardial Fibrosis (EMF) was considered as a differential diagnosis potentially due to giant cell myocarditis, an atypical form of infection such a malaria or toxoplasmosis or exposure to toxic agents. Blood smear for Malaria was reported negative and Echinococcus IgG (to rule out hydatid cyst disease) was negative.
Patient was discharged on optimal medical therapy as listed previously, along with lifelong anticoagulation with warfarin. Patient was advised to undergo endomyocardial biopsy for further assessment of EMF, however, he declined further investigation. Unfortunately, the patient left the country after receiving treatment, making continued monitoring and retrospective analysis challenging.
Discussion:
Left ventricular noncompaction (LVNC) was first pathologically identified by Dusek in 1975 and later described via 2D echocardiography by Engberding and Bender in 1984 [5].Diagnosing and managing LVNC presents significant challenges due to its wide clinical variability. It is difficult to distinguish LVNC from hypertrophic and dilated cardiomyopathies due to similarities in its characteristics. There is an ongoing debate about whether LVNC is a separate condition or a morphological variation among several cardiomyopathies, with the European Society of Cardiology classifying it as an unclassified cardiomyopathy and the World Health Organization classifying it as a primary cardiomyopathy [6]. In LVNC, the left ventricular myocardium, particularly in the distal region, appears spongy, characterized by deep intertrabecular recesses (or sinusoids) that communicate with the left ventricular cavity, along with prominent ventricular trabeculations [7].
To understand the pathophysiology of LVNC, it is essential to focus on embryogenesis. Similar patterns of complex alterations in myocardial maturation are seen during the development of the heart in animals, including humans. Early heart tube construction, trabeculation emergence, trabecular remodeling, and the formation of a multilayered spiral system are the four stages in the evolution of the myocardial architecture.Key stages for understanding left ventricular noncompaction (LVNC) are the emergence of trabeculations and trabecular remodeling [8].
In human embryos, trabeculations appear after the primitive heart tube loops at the end of the fourth gestational week. These early trabeculations increase the myocardial surface area, enabling the myocardial mass to grow before the development of an epicardial coronary circulation. At this stage, trabeculations in the left ventricle (LV) are typically thicker, and the intertrabecular spaces are larger. Persistent trabeculation patterns postnatally resemble the spongy myocardium of nonmammalian vertebrates [1].
The trabecular remodeling process begins after ventricular septation completes around the eighth week of gestation. Ventricular volume increases, compressing trabeculations and thickening the compact myocardium. The remodeling is specific to ventricles and species; in mammals, luminal trabeculations coalesce to form structures like the papillary muscles of the mitral valve, while apical trabeculations transform into fine honeycomb-like patterns on the inner ventricular surface. Trabecular remodeling coincides with the invasion of coronary arteries and myocardial vascularization, progressing from the epicardium to the endocardium, base to apex, and septum to the free wall, more pronounced in the LV than in the right ventricle [8].
This brings us to the question of whether LVNC is congenital or acquired. LVNC is generally considered to reflect impaired or arrested myocardial compaction during development [9]. However, there is debate over whether LVNC can also be acquired, as some echocardiographic studies show LVNC emerging later in life [10]. Genetic evidence suggests a possible postnatal development of LVNC, similar to dilated and hypertrophic cardiomyopathies, which share common mutations in sarcomere protein genes with LVNC. This raises the hypothesis that LVNC may also develop due to genetic factors later in life [1].
LVNC can occur in isolation or in association with congenital heart or neuromuscular diseases [6]. The Heart Rhythm Society states that genetic testing is recommended (Class I) for relatives and appropriate family members when a mutation-specific gene has been identified [11].
The diagnosis of LVNC primarily relies on echocardiography that is often the first-line imaging modality. Three main diagnostic criteria for LVNC, developed by Chin et al., Stöllberger and Finsterer, and Jenni. Chin’s criteria measures LVNC in end systole while Jenni’s criteria measures it in end diastole. Chin et al.'s criteria [10], includes measuring the left ventricular (LV) free-wall thickness and the ratio of myocardial thickness at different points. Stöllberger and Finsterer’s criteria [10], require the presence of more than three trabeculations and a two-layered myocardium, however this criteria may lead to overdiagnosis. The Jenni criteria [4] that are widely used include the presence of bilayered myocardium with a noncompacted to compacted ratio greater than 2:1, trabeculations, and communication with intertrabecular spaces without other cardiac abnormalities [10].
Echocardiography has limitations, such as challenges in imaging the apex and potential inaccuracies with off-axis images. Skilled sonographers and the use of contrast can mitigate some of these issues. When echocardiographic findings are indeterminate or when high image quality is necessary, Cardiac Magnetic Resonance Imaging (CMR) is a useful secondary option [5].
CMR provides high-resolution imaging, better differentiation of myocardial layers, and the ability to use late gadolinium enhancement for assessing fibrosis [12]. According to the Petersen criteria, an NC/C ratio >2.3 in end-diastole is diagnostic for LVNC, the Jacquier criteria require trabeculated LV mass >20% of the global mass. Choi criteria include an NC/C ratio >2.3 in short-axis views during end-diastole, assessed with specific volumetric analysis software. The Petersen criteria are the most widely used, as they can distinguish pathological LVNC with high specificity and sensitivity [13].
Cardiac Computed Tomography (CT) is valuable tool for patients with newly diagnosed heart failure who require both LVNC evaluation and coronary artery disease exclusion. While CT provides good spatial resolution for LVNC identification, its high radiation exposure and the potential for contrast dye reactions limit its routine use. CT can be used complementarily when echocardiography and CMR results are equivocal or when coronary artery disease also needs evaluation [14].
Reducing thromboembolic risk, avoiding arrhythmias, and treating heart failure symptoms are the main goals of LVNC management [15]. Since all three components of Virchow's Triad—hypercoagulability, blood flow stasis, and endothelial injury—that lead to the development of LV thrombus are present in LVNC, anticoagulation therapy is the mainstay of treatment for patients with LVNC. DOACs are the first choice for anticoagulation that is utilized when the ejection fraction (EF) is less than 40% or if thromboembolic events have occurred in the past [16]. Oral anticoagulation is advised for patients with concomitant arrhythmias, especially atrial fibrillation. In patients with EF ≤ 35%, arrhythmia therapy may include ICD installation in addition to anticoagulation to prevent sudden death [17].
Sudden cardiac arrest is a rare but serious presentation of LVNC, often associated with high mortality rates. Studies have shown that syncope and seizures may be warning signs for life-threatening arrhythmias in LVNC patients [18].
Diuretics, ACE inhibitors, ARBs, beta blockers, sacubitril-valsartan, and sodium-glucose Cotransporter-2 (SGLT2) inhibitors are used to treat congestive heart failure in LVNC; in extreme situations, cardiac transplantation may be necessary [19].
Additionally, it was found that our patient had endomyocardial fibrosis (EMF), a kind of endemic restrictive cardiomyopathy that primarily affects children and adolescents and is common in impoverished areas of Asia, Latin America, and Africa [20].
Our patient was also discovered to have Endomyocardial fibrosis (EMF), which is a form of endemic restrictive cardiomyopathy prevalent in poor regions of Africa, Latin America, and Asia, affecting mainly children and adolescents [20]. It accounts for up to 20% of heart failure cases in some African regions [21]. The exact cause and mechanisms of EMF remain unclear, though various factors such as ethnicity, poverty, diet, infections, and genetic predisposition are implicated [22]. Clinically, EMF causes endocardial fibrosis and reduction in the size of ventricular cavity that results in restrictive filling and severe atrioventricular regurgitation [21]. The disease progression is poorly understood due to late presentation and lack of research infrastructure in endemic areas [22]. Echocardiography is the diagnostic modality of choice when making the diagnosis of EMF. Findings from endomyocardial biopsy may be diagnostic, but this procedure is typically not needed. Biopsy findings may be non-diagnostic when the disease is patchy and sampling sites do not correlate with the areas of disease. Mocumbi and colleagues provided a set of echocardiographic criteria that is helpful for diagnosing as well as staging the disease and to study its progression [23]. Surgical intervention has shown to improve survival and quality of life [22].
Despite advancements, the prognosis remains poor, with high rates of mortality and recurrent disease. Further research and resources are crucial to better understand and combat this neglected cardiovascular disease [22].
Conclusion:
Left Ventricular Non-Compaction (LVNC) is a rare cardiomyopathy resulting from incomplete myocardial compaction during embryogenesis. It poses diagnostic challenges due to its overlap with other cardiomyopathies, requiring advanced imaging techniques for accurate identification. Management focuses on heart failure treatment, thromboembolism prevention, and arrhythmia control, often necessitating lifelong anticoagulation. This case highlights the importance of recognizing LVNC in heart failure patients, the role of genetic evaluation, and the need for further research to enhance prognosis and personalized treatment strategies
References
-
Lorca R, Pascual I, Fernandez M, Alvarez-Velasco R, Colunga S, Muñiz M, Izquierdo M, Fernandez Y, Esteban E, Gomez J, Avanzas P, Lopez-Fernandez T. (2023) Concealed Inherited Cardiomyopathies Detected in Cardio-Oncology Screening. J Clin Med. Dec 19;13(1):2. doi: 10.3390/jcm13010002; PMID: 38202009; PMCID: PMC10780282
View at Publisher | View at Google Scholar -
Akinseye OA, Ibebuogu UN, Jha SK. (2017) Left Ventricular Noncompaction Cardiomyopathy and Recurrent Polymorphic Ventricular Tachycardia: A Case Report and Literature Review. Perm J. 21:17-045. doi: 10.7812/TPP/17-045. PMID: 29035186; PMCID: PMC5638626.
View at Publisher | View at Google Scholar -
Fitzsimons LA, Kneeland-Barber DM, Hannigan GC, Karpe DA, Wu L, Colon M, Randall J, Tucker KL. (2024) Electrophysiological phenotyping of left ventricular noncompaction cardiomyopathy in pediatric populations: A systematic review. Physiol Rep. 12(9):e16029. doi: 10.14814/phy2.16029. PMID: 38684446; PMCID: PMC11058051.
View at Publisher | View at Google Scholar -
Ahmed SA, Karataş M, Öcal L, Hassan MS, Mohamud MA, Hassan MO, Dirie AMH, Waberi MM, Ali AA. (2022) Isolated left ventricular non-compaction cardiomyopathy complicated by acute ischemic stroke: A rare case repor. Ann Med Surg (Lond). 81:104543. doi: 10.1016/j.amsu.2022.104543. PMID: 36147147; PMCID: PMC9486750.
View at Publisher | View at Google Scholar -
Bilal MI, Ansari FA, Gondal MUR, Aftab M, Qureshi AM, Kassis-George H. (2024) Exploring the Unknown: Appreciating the Challenges of Non-compaction Cardiomyopathy. Cureus. 16(5):e61142. doi: 10.7759/cureus.61142. PMID: 38933642; PMCID: PMC11199402.
View at Publisher | View at Google Scholar -
Hirono K, Hata Y, Miyao N, Okabe M, Takarada S, Nakaoka H, Ibuki K, Ozawa S, Yoshimura N, Nishida N, Ichida F, Lvnc Study Collaborators. (2020) Left Ventricular Noncompaction and Congenital Heart Disease Increases the Risk of Congestive Heart Failure. J Clin Med. 9(3):785. doi: 10.3390/jcm9030785. PMID: 32183154; PMCID: PMC7141335.
View at Publisher | View at Google Scholar -
Barati Z, Farhoud D, Nixdorff U, Mohammadhasani M, Eslami M, Nayernia K. (2021) A Case Report of Genetic Cascade Screening in Dilated Cardiomyopathy: A Perspective for Preventive Cardiology. Iran J Public Health. 50(12):2593-2598. doi: 10.18502/ijph.v50i12.7943. PMID: 36317039; PMCID: PMC9577170.
View at Publisher | View at Google Scholar -
Trinidad F, Rubonal F, Rodriguez de Castro I, Pirzadeh I, Gerrah R, Kheradvar A, Rugonyi S. (2022) Effect of Blood Flow on Cardiac Morphogenesis and Formation of Congenital Heart Defects. J Cardiovasc Dev Dis.;9(9):303. doi: 10.3390/jcdd9090303. PMID: 36135448; PMCID: PMC9503889.
View at Publisher | View at Google Scholar -
Petersen SE, Jensen B, Aung N, Friedrich MG, McMahon CJ, Mohiddin SA, Pignatelli RH, Ricci F, Anderson RH, Bluemke DA. (2023) Excessive Trabeculation of the Left Ventricle: JACC: Cardiovascular Imaging Expert Panel Paper. JACC Cardiovasc Imaging. (3):408-425. doi: 10.1016/j.jcmg.2022.12.026. Epub 2023 Feb 8. PMID: 36764891; PMCID: PMC9988693.
View at Publisher | View at Google Scholar -
Lutokhina Y, Blagova O, Nedostup A, Alexandrova S, Shestak A, Zaklyazminskaya E. (2020) Clinical Classification of Arrhythmogenic Right Ventricular Cardiomyopathy. Pulse (Basel). 2020 Aug;8(1-2):21-30. doi: 10.1159/000505652. Epub PMID: 32999875; PMCID: PMC7506253.
View at Publisher | View at Google Scholar -
Chao T, Ge Y, Sun J, Wang C. (2024) Research landscape of genetics in dilated cardiomyopathy: insight from a bibliometric analysis. Front Cardiovasc Med. 11:1362551. doi: 10.3389/fcvm.2024.1362551. PMID: 39070560; PMCID: PMC11272475.
View at Publisher | View at Google Scholar -
Kiss AR, Gregor Z, Furák Á, Szabó LE, Dohy Z, Merkely B, Vágó H, Szűcs A. (2021) Age- and Sex-Specific Characteristics of Right Ventricular Compacted and Non-compacted Myocardium by Cardiac Magnetic Resonance. Front Cardiovasc Med. 8: 781393. doi: 10.3389/fcvm.2021.781393. PMID: 34950717; PMCID: PMC8688768.
View at Publisher | View at Google Scholar -
Grebur K, Mester B, Horváth M, Farkas-Sütő K, Gregor Z, Kiss AR, Tóth A, Kovács A, Fábián A, Lakatos BK, Fekete BA, Csonka K, Bödör C, Merkely B, Vágó H, Szűcs A. (2024) The effect of excessive trabeculation on cardiac rotation-A multimodal imaging study. PLoS One. 19(9):e0308035. doi: 10.1371/journal.pone.0308035. PMID: 39236040; PMCID: PMC11376564.
View at Publisher | View at Google Scholar -
Ogah OS, Iyawe EP, Okwunze KF, Nwamadiegesi CA, Obiekwe FE, Fabowale MO, Okeke M, Orimolade OA, Olalusi OV, Aje A, Adebiyi A. (2023) LEFT VENTRICULAR NONCOMPACTION CARDIOMYOPATHY: A SCOPING REVIEW. Ann Ib Postgrad Med. 2023 Aug;21(2):8-16. Epub PMID: 38298349; PMCID: PMC10811705.
View at Publisher | View at Google Scholar -
Ritchie C, Albagoush S. (2022) Left Ventricular Non-compaction in a 40-Year-Old Male With Congenital Hydrocephalus. Cureus. 14(9):e29546. doi: 10.7759/cureus.29546. PMID: 36312646; PMCID: PMC9592865.
View at Publisher | View at Google Scholar -
Chimenti C, Lavalle C, Magnocavallo M, Alfarano M, Mariani MV, Bernardini F, Della Rocca DG, Galardo G, Severino P, Di Lullo L, Miraldi F, Fedele F, Frustaci A. (2022) A proposed strategy for anticoagulation therapy in noncompaction cardiomyopathy. ESC Heart Fail. 9(1):241-250. doi: 10.1002/ehf2.13694. Epub 2021 Dec 16. PMID: 34918480; PMCID: PMC8788052.
View at Publisher | View at Google Scholar -
Alrefaee A, Wiseman K, Udongwo N, Sathya B, Demchuk B. (2022) Ventricular Noncompaction With Left Ventricular Thrombus: A Case Report. Cureus. 14(6):e25605. doi: 10.7759/cureus.25605. PMID: 35795525; PMCID: PMC9250348.
View at Publisher | View at Google Scholar -
Tasha T, Ezeh E, Sonani N (2022) An Unusual Presentation of Left Ventricular Non-compaction Cardiomyopathy in a Female Patient With Sudden Cardiac Arrest: A Case Report. Cureus 14(10): e30830. doi:10.7759/cureus.30830
View at Publisher | View at Google Scholar -
Gerecke BJ, Engberding R. (2021) Noncompaction Cardiomyopathy-History and Current Knowledge for Clinical Practice. J Clin Med. 10(11):2457. doi: 10.3390/jcm10112457. PMID: 34206037; PMCID: PMC8199228.
View at Publisher | View at Google Scholar -
Tirfe M, Nedi T, Mekonnen D, Berha AB. (2020) Treatment outcome and its predictors among patients of acute heart failure at a tertiary care hospital in Ethiopia: a prospective observational study. BMC Cardiovasc Disord. 20(1):16. doi: 10.1186/s12872-019-01318-x. PMID: 31959121; PMCID: PMC6971982.
View at Publisher | View at Google Scholar -
Duraes AR, de Souza Lima Bitar Y, Roever L, Neto MG. (2020) Endomyocardial fibrosis: past, present, and future. Heart Fail Rev. 25(5):725-730. doi: 10.1007/s10741-019-09848-4. PMID: 31414216.
View at Publisher | View at Google Scholar -
Aliku TO, Rwebembera J, Lubega S, Zhang W, Lugero C, Namuyonga J, Omagino JOO, Okello E, Lwabi PS. (2022) Trends in Annual Incidence Rates of Newly Diagnosed Endomyocardial Fibrosis Cases at the Uganda Heart Institute: A 14-Year Review. Front Cardiovasc Med. 9:841346. doi: 10.3389/fcvm.2022.841346. PMID: 35498040; PMCID: PMC9051226.
View at Publisher | View at Google Scholar -
Minja NW, Nakagaayi D, Aliku T, Zhang W, Ssinabulya I, Nabaale J, Amutuhaire W, de Loizaga SR, Ndagire E, Rwebembera J, Okello E, Kayima (2022) J. Cardiovascular diseases in Africa in the twenty-first century: Gaps and priorities going forward. Front Cardiovasc Med. 9:1008335. doi: 10.3389/fcvm.2022.1008335. PMID: 36440012; PMCID: PMC9686438.
View at Publisher | View at Google Scholar






