Research Article | DOI: https://doi.org/10.5281/zenodo.14942872
A Novel Dissolution Method for Simultaneous Estimation of Sennoside A and Sennoside B in Senna Tablet
Abstract
The current method was developed for the small-scale herbal industries established in India or the presented sectors in other countries and for the regulatory interest to amend the Senna tablet monograph stated in Indian Pharmacopoeia. The existing method for the dissolution of Senna tablets was cost-consuming and unsuitable for herbal small-scale industries to assay Senna tablets. The present study indicates a reliable high-performance liquid chromatographic method for quantifying sennoside A and sennoside B in commercial Senna tablets. The separation of sennoside A and sennoside B was conducted on a Hypersil C 18 column (150 × 4.6mm, 3.5µm) at a temperature of 40°C, using a mixture of 75 volumes of 1 percent v/v solution of glacial acetic acid and 25 volumes of acetonitrile containing 10µl tetra n-butyl ammonium hydroxide (ion-pairing reagent) used as mobile phase. The current method was developed and validated. The sennoside A and sennoside B were completely separated from other constituents within 15 minutes. Both run-to-run repeatability (n=10) and day-to-day reproducibility (n=3) of peak area were below 0.5% RSD. The linearity of peak area was tested in the ranges 18-32µg/ml (r˃0.9935). Accuracy and recovery were assessed and the recoveries for sennoside A and sennoside B were 100.2% and 104.9% (n=3×6), respectively. Thus, the developed method is simple, accurate, and precise and may be adopted by the pharmacopoeial bodies as an official method. It is also suitable for small-scale herbal industries.
Introduction:
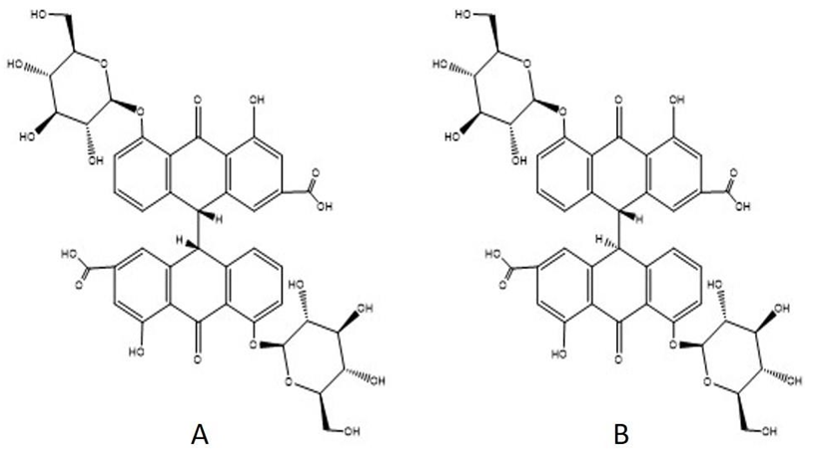
The genus Senna includes 500 species, with 26 species of the genus Cassia containing anthracene derivatives withinside the unfastened shape or glycosides. Cassia angustifolia (Indian Senna) and Cassia acutifolia (Alexandrian Senna) are official in many pharmacopeias because of their laxative activity and availability in considerable numbers. C. fistula, C. alata, C. dentate, C. obovata, C. podocarpa, C. sieberiana, and C. sofara are the other species with documented laxative action. Senna is a medication that is used as a laxative and for treating constipation and bowel evacuation. Senna is used before the surgery to clear the large intestine. The active ingredients of Senna are anthraquinone glycosides (sennoside A, B, C, and D), the free anthraquinones are rhein, chrysophanol, etc. from the active ingredients present in Senna, sennoside A and sennoside B (Figure 1) were exist in the maximum amount [1]. Senna tablet is generally given by mouth and by rectum. When ingested by mouth, Senna preparation works in twelve hours and within some minutes when introduced through the rectum. Dissolution analysis of herbal dosage forms has become the most crucial test to ensure product quality [2]. The disintegration or dissolution test is generally seen during the analysis of allopathic solid dosage form because it will determine the absorption rate after dissolving in gastric fluid, and ultimately, it will define the bioavailability of the drug [3].
Keeping the same concept in mind, the dissolution analysis for the Senna tablet is to be carried out to assess the release rate and drug bioavailability. While different pharmacopoeias stated in the monograph on Senna tablets like USP, IP, and The Ayurvedic Pharmacopoeia of India to date, the HPLC dissolution method for the estimation of sennoside A and sennoside B in Senna tablets is not formally established and validated in any pharmacopoeias except the United State Pharmacopoeia. Among the various techniques available for the development of the assay method, UV/visible spectrophotometry achieves the advantage of low cost and simplicity with the possibility of achieving high sensitivity and selectivity with reasonable accuracy, precision, and reliability [4] [5] [6].
In India, most herbal industries were established as small-scale industries. These industries cannot implement a method that includes the usage of a cost-consuming detector, such as assaying by fluoroscopy technique. Also, some need was to be captured for regulatory implantation in the Indian Pharmacopoeia (IP) [4].
The effect of sennoside A and sennoside B is most significant in the Senna drug [7]. Therefore, it is essential to screen out the quantity of sennoside A and sennoside B by dissolving the Senna tablet [8] [9]. To resolve the theory, an accurate, precise method was essential to develop and validate.
The developed method helps to govern the regulatory amendment of dissolution studies of herbal solid dosage forms to be established in the monograph of Senna tablet stated in Indian Pharmacopoeia but also provides a new concept to various herbal small-scale industries by accepting a precise and efficient method for the assay of Senna tablets by HPLC.
2. Material and Methods
2.1 Chemicals and Reagents:
Sennoside A (>96.0%) and sennoside B (>90.0%) standards were procured from Sigma –Aldrich (Germany), Senna tablets (12 mg) were procured from market, Glacial Acetic Acid, Sodium hydroxide pellets, and HCl were purchased from Avantor, Tetra n-butyl ammonium hydroxide 0.1N aqueous solution LR was purchased from S. D. fine chemical ltd., Acetonitrile of chromatographic grade was purchased from Merck India Ltd. and Hydrogen Peroxide was purchase from Qualikems Pvt. ltd.
2.2 Chromatographic equipment and conditions:
For quantification of sennoside A and sennoside B, an Agilent 1260 series HPLC instrument (Agilent Technologies, USA) composed of an online degasser (G1322A), a quaternary pump (G1311C), an auto-Sampler (G1329B), a column temperature controller (G1316A), and a DAD (G1315D) and Dissolution test apparatus (Lab India DS 8000) was used. Analysis was carried out on an Agilent TC-C18 reverse-phase column (150× 4.6 mm, 3.5 µm). A mixture of 75 volumes of 1 per cent v/v of glacial acetic acid, 25 volumes of acetonitrile v/v, and 10 µl of tetra n-butyl ammonium hydroxide was used as a mobile phase. The method was developed with a flow rate of 0.5 ml/min, injection volume of 10 µl, detection wavelength at 350 nm, and column temperature at 40°C.
2.3 Preparation of standard solutions:
Sennoside A and sennoside B standard solutions were prepared by dissolving 12 mg of the respective compounds in 10 ml of water. After filtration through 0.45 µm Nylon membranes, the standard solutions were used to make calibration curves.
2.4 Sample Preparation:
Twenty tablets from five batches of 12 mg of Senna tablets were taken from the market and ground to make a fine powder. 12 mg of the powder was dissolved in 12 ml of water (0.005 mg in 900 ml). The mixture was sonicated to make sennoside A and sennoside B completely dissolve. After filtration through a 0.45 µm Nylon membrane, the solution was used as a working solution in method development.
2.5 Method validation
The dissolution was validated according to ICH Q2 (R2) guidelines for validating analytical procedures. The method was validated using the isocratic phase, which is simpler and more stable than a gradient system and, thus, more suitable for QC and routine analysis.
3. Results
3.1 Chromatography and detection
The reverse-phase high-performance liquid chromatography (RP-HPLC) detected sennoside A and sennoside B in standards and samples. Sennoside A and sennoside B were separated within 15 minutes at 350 nm as shown in (Figure 2).
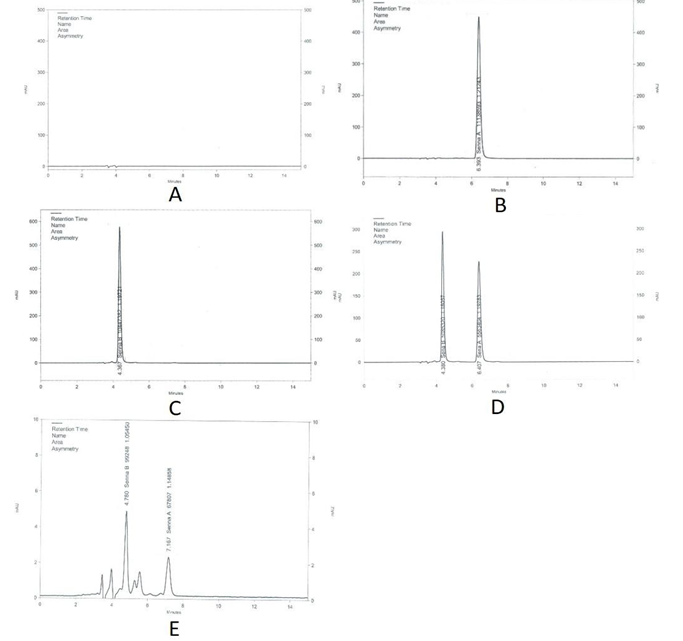
3.1.1 Specificity and Selectivity:
The developed method was selective for sennoside B and sennoside A. The retention time of sennoside B and sennoside A is 4.3 and 6.2 minutes, respectively. At the retention time of sennoside A and sennoside B, when examined at a wavelength of 350 nm, the chromatogram of the blank (Figure 2A) confirmed the absence of interfering peaks. The result obtained from the chromatogram of sennoside A and sennoside B (Figure 2 B, 2 C) demonstrates no interference from other materials in the developed method and therefore confirms the method's specificity.
3.1.2 Accuracy and precision:
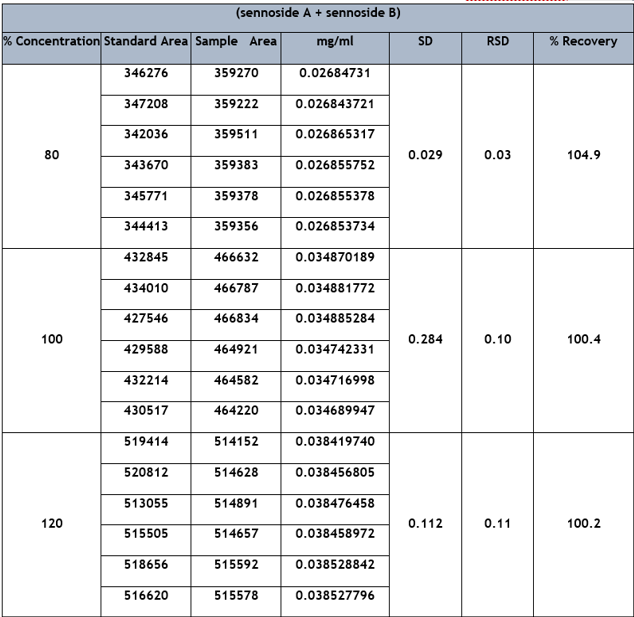
Run-to-run repeatability and day-to-day reproducibility were used to assess the precision of repeated injections. Six injections were made each day and repeated for 3 consecutive days, as shown in Table 1. The relative standard deviation (RSD) of the retention time of sennoside A and sennoside B is 0.55%. The sample solutions were prepared to contain sennoside A and sennoside B in three concentration ranges 80%, 100%, and 120% by spiking the standard solution in the respective range. The recoveries obtained with six repetitive injections are 100.2%, 100.4%, and 104.9%. To test the repeatability and reproducibility of the recoveries, the above procedure was repeated six times a day for three consecutive days. The RSD of recovery of sennoside A and sennoside B were 0.11, 0.10, and 0.03 for concentration 120%, 100%, and 80% respectively, as shown in Table 2.
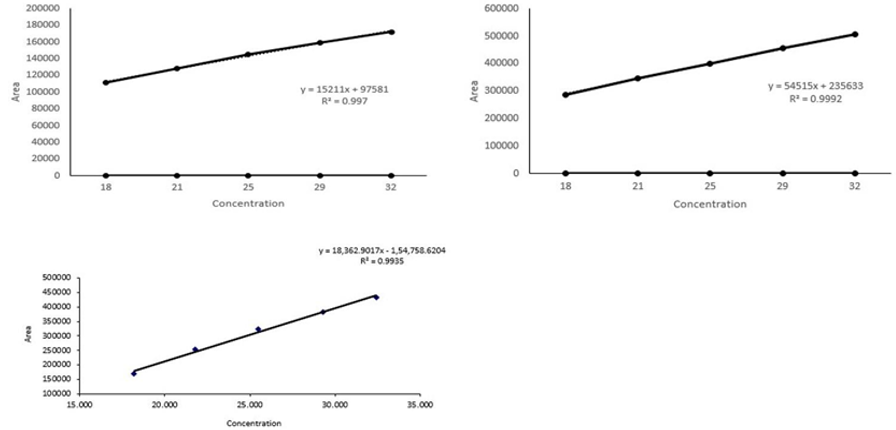
3.1.3 Linearity
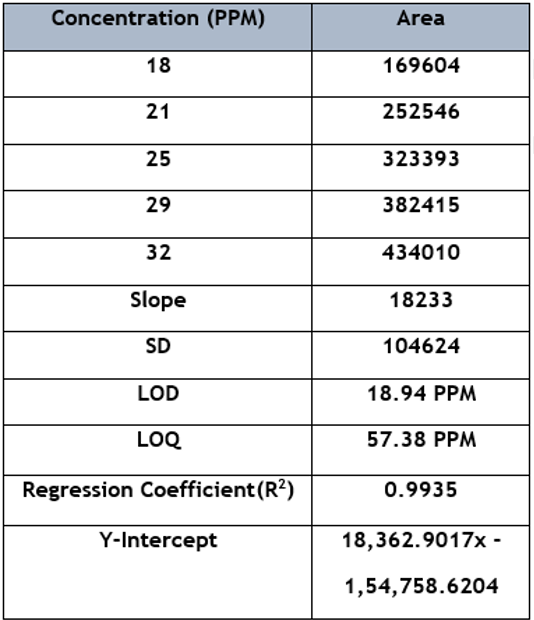
Five standard solutions (18, 21, 25, 29, and 32 µg/ml) were run for the calibration curve. On each day of three consecutive days, the calibration curves were made. Slopes, y-intercepts, and correlation coefficient (r2) were calculated. The calibration curves were found linear over 18-32 µg/ml. Their coefficient of correlation (r) was all above 0.99. The equations were y =18,362.9017x - 1, 54,758.6204 and for sennoside A and sennoside B, respectively [y for peak area and x for concentration in (g/ml) as shown in (Figure 3)]. After passing the statistical test three proved no difference, the three lines were combined to form a new line for quantitative use.
3.1.4 Robustness
The effect of each factor was calculated. Based on these data, the effects of the factors were discussed [10] [11].
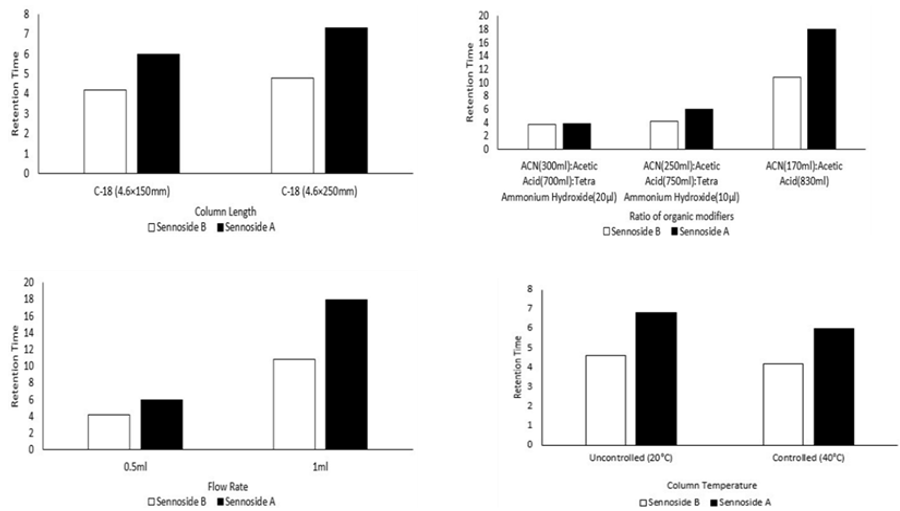
3.1.4.1 Effect of columns
Resolution was affected by the change of columns, but the effect was unidirectional (Figure 4A); only when the column was significant. No matter what column was used, the retention time of sennoside A was greatly influenced (Figure 4A). This is in accordance with our previous observation that the symmetry and Luna column retained the sennoside far more than the Hypersil column.
3.1.4.2 Ratio of organic modifiers
The change in the percentage of acetonitrile in the mobile phase greatly influenced both resolution and retention time. Its lessening from (30 to 25% v/v) helped the resolution to the greatest extent (Figure 5 B), however, at the expense of terrible prolongation of retention time. Because the plate number of sennoside A was affected by the change (Figure 5 B), this might have something to do with the change in retention time.
3.1.4.3 Effect of flow rate
The peak efficiency of sennoside A was significantly affected by the change in mobile phase flow rate, when the flow rate was slowed from 1 ml/min to 0.5 ml/min. This is because the content of sennoside A lowers the sennoside B for quantifying the sennoside A. The flow rate of the mobile phase shifted to 0.5 ml/min, as shown in (Figure 5 D).
3.1.4.4 Effect of column temperature
The change in column temperature from 20°C to 40°C shows the change in the retention time of sennoside A and sennoside B. So, the method was developed at 40°C to achieve the desired retention time and high plate numbers.
Limit of detection and Limit of quantification (LOD & LOQ)
LOD and LOQ were obtained for sennoside A and sennoside B, which cause a peak height three times and ten times the height of baseline noise, respectively [12] [13]. According to earlier parameters, LOD and LOQ were estimated to be 18.94 ppm and 57.38 ppm, respectively, as shown in Table 3.
3.1.6 Stability of solutions
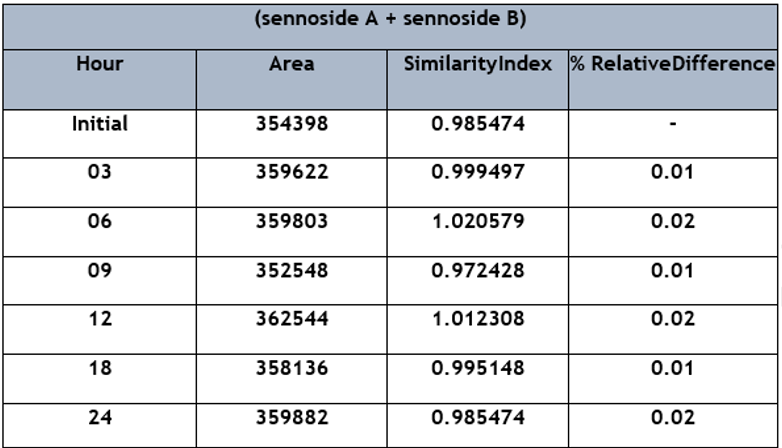
The stability of solutions was tested with standard solutions that were stored at 4°C and room temperature (about 25°C) for 24 hours. After each storage condition, the concentration of sennoside A and sennoside B was analyzed, and the results were compared to freshly prepared samples, as shown in Table 4.
3.1.7 Force Degradation
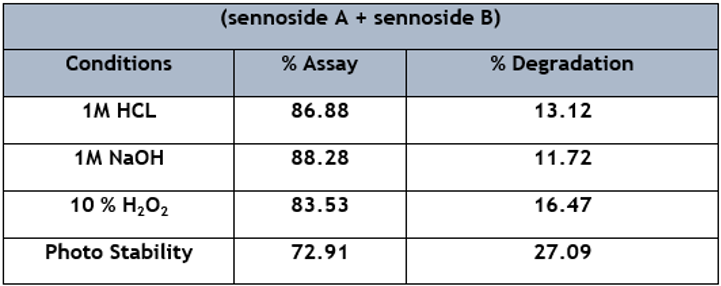
The sample of Senna tablets was provided with an addition of external stress, and the sample was admixed with 1 M HCL (Acid), 1 M NaOH (Alkali), and 10% H2O2 (Oxidation) separately; the observed degradation was found to be 13.12%, 11.72%, and 16.47% respectively. The sample was also tested for photo stability and kept in light for 24 hours. After 24 hours, the sample was analysed and degraded by 27.09%, as shown in Table 5.
3.2. Statistical Analysis
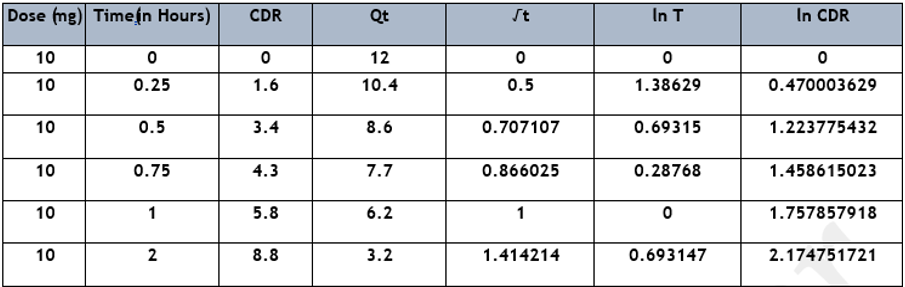
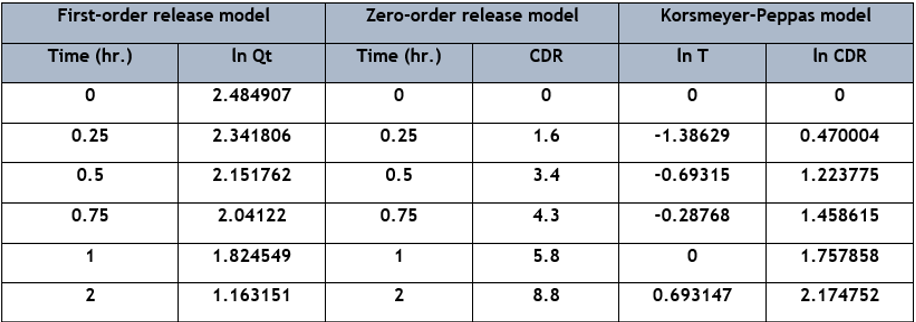
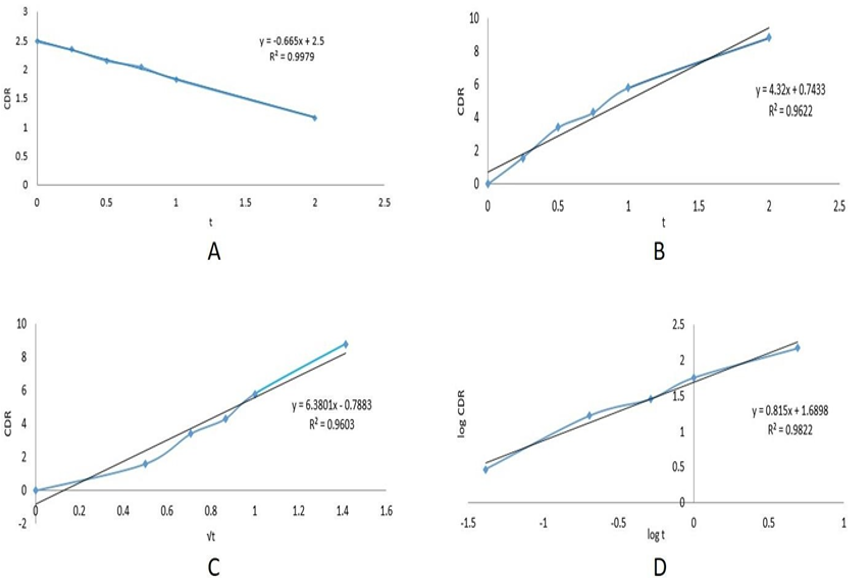
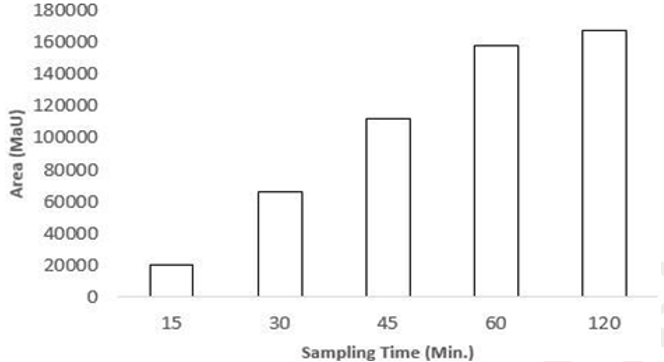
Data analysis was performed by using the formula (Average area of sample /average area of standard × weight of standard used /dilution of standard solution × dilution of sample solution /weight of sample × average weight /label claim × potency) and by the implementation of various statistical models (First-order kinetics, Zero-order kinetics, Korsmeyer–Peppas model, and Higuchi model) for assessing the releasing rate of Senna tablet, from which some models were described below as shown in Table 6, Table 7, and (Figure 5) [14] [15] [16]. The release pattern of the Senna tablet follows the first-order kinetics model that is used to define the elimination and absorption of samples. The elevation of the releasing of the Senna tablet concerning time is shown in (Figure 6), and the obtained records are designed between log cumulative percentage of remaining drug versus time that will provide a straight line, and the slope will be given as -K/2.303 as shown in (Figure 6 A). The rest of the models observed were displayed in (Figure 5).
3.3. Assay of the tablets
The average weight of a tablet is 139.3 ± 5.0 mg. The tablet contains 1.93 ± 0.01 mg (n=3) of sennoside A and 5.44 ± 0.03 mg (n=3) of sennoside B. The tablet's combined weight of sennoside A and sennoside B is 7.37 ± 0.02 mg (n=3).
Discussion:
The developed method was validated for the estimation of sennoside A and sennoside B in the Senna tablet by the RP-HPLC method. [17] [18] The current method was developed with the help of a Hypersil C-18(150× 4.6 mm, 3.5µm) column, using the mixture of 75 volumes of a 1 percent v/v of glacial acetic acid and 25 volumes of acetonitrile v/v and 10 µl of tetra n- butyl ammonium hydroxide was used as a mobile phase. The flow rate was kept at 0.5ml per min. for the optimum analysis condition. An injection volume of 10µl was used to inject the sample. The detection was done using a DAD detector with the ʎmax of 350 nm. The time of retention of peaks was found to be at 4.3 and 6.2 minutes for sennoside B and sennoside A, respectively.
The linearity range was set at 18-32 µg/ml; where a good resolution and peak shape were found with a good correlation coefficient value. The recovery was done at three different levels 80%, 100%, and 120%. The method was found entirely suitable, the precision was carried out by repeated injection of the same sample, and the SD was found to be 104624. The intra-day and inter-day precision was also carried out and the percentage RSD was found to be less than 2%. The robustness of the developed method was countered by variations in column size, ratio of organic modifiers, flow rate, and column temperature [19] [20]. The forced degradation study of the sample was also analyzed by providing some external stress to the sample with 1M HCL (Acid), 1M NaOH (Alkaline), 10 % H2O2 (Oxidation), and for photostability, and the results were found in an acceptable range. The sample was analyzed for stability by maintaining the standards at a temperature of 25°C for 24 hours, and no significant variations were found in the concentration of sennoside A and sennoside B. The LOD and LOQ are 18.94 and 57.38 µg/ml respectively. The method validation results of all nine factors are satisfactory limits.
This method is fully compatible with resolving both principal compounds sennoside A and sennoside B within 15 minutes of retention time. Further, the developed method was utilized to assay the sennoside A and sennoside B in the Senna tablet. The dissolution apparatus was fully calibrated according to the dissolution conditions for the USP type 1 apparatus. HPLC-grade water with a pH of 7.0 was used as the dissolution media, and the temperature was maintained at 37 ± 0.5°C with 100 rotations per minute. Samples were withdrawn from the dissolution apparatus at 15, 30, 45, 60, and 120 minutes while maintaining sink conditions, as shown in Figure 6. The retention times of sennoside A and sennoside B were consistent with those obtained during validation. Then, the statistical calculation was done by implantation of the various mathematical models. Based on these models, the drug release rate of the Senna tablet follows first-order kinetics. This is the best condition for any tablet to release the drug concerning the time. Hence, the developed method based on RP-HPLC can be utilized to assay the Senna tablet by dissolution method, and improvement of the quality of the drug during production can be maintained.
Conclusion:
The current work describes the developed method for the estimation and determination of sennoside A and sennoside B in standards and Senna tablets on RP-HPLC. Based on the literature survey, we have decided to develop a new method that would be fast, economical, and reliable. The method was validated leading by the ICH guidelines. The sennoside A and sennoside B both were resolved on Hypersil C-18 (150× 4.6 mm, 3.5µ) column using the mixture of 75 volumes of a 1 percent v/v of glacial acetic acid and 25 volumes of acetonitrile v/v and 10 µl of tetra n- butyl ammonium hydroxide as mobile phase and on a flow rate of 0.5ml/min., and the ʎmax was set at 350 nm. The percentage RSD for the tablet analysis was less than 2%. The percentage RSD of sennoside A and sennoside B for injection reproducibility and interday precision was < 2>
The developed method not only helps govern the regulation of dissolution of herbal solid dosage forms to be established in Indian pharmacopeia as established in United States Pharmacopoeia but also provides a new concept to various herbal small-scale industries by accepting a precise and efficient method for the assay of Senna tablets by HPLC.
Acknowledgement:
The authors acknowledge Dr. Rajeev Singh Raghuvanshi, Drug Controller General of India (DCGI), Central Drug Standard Control Organization (CDSCO), Secretary-cum-Scientific Director, Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Government of India and Dr. Gaurav Pratap Singh Jadaun, Senior Principal Scientific Officer, Head of Departments of Analytical Research & Development (AR&D) and Phytopharmaceuticals, Indian Pharmacopoeia Commission, Ministry of Health and Family Welfare, Government of India, for the platform to develop the manuscript.
Conflict of interests
All authors declare that they have no conflicts of interest.
Statement of ethics
Not applicable.
Availability of data and materials
Not applicable.
Funding :
None
References
-
Lal, R.K., Chanotiya, C.S. and Kumar, A., (2023) The prospects and potential of the horticultural and pharmacological medicinal herb senna (Cassia angustifolia Vahl.): a review. Technology in Horticulture, 3(1).
View at Publisher | View at Google Scholar -
Hasan, M.M., Rahman, M.M., Islam, M.R., Hasan, H., Hasan, M.M. and Rashid, H.A., (2017). A key approach on dissolution of pharmaceutical dosage forms. Pharma Innov. J, 6, pp.168-180.
View at Publisher | View at Google Scholar -
Braithwaite, M.C., Tyagi, C., Tomar, L.K., Kumar, P., Choonara, Y.E. and Pillay, V., (2014). Nutraceutical-based therapeutics and formulation strategies augmenting their efficiency to complement modern medicine: An overview. Journal of Functional Foods, 6, pp.82-99.
View at Publisher | View at Google Scholar -
Indian Pharmacopoeia (2022). The Indian Pharmacopoeia Commission, Ministry of Health & Family Welfare, Government of India, Ghaziabad. 9th Edition, Vol–III, p. 4291-4292.
View at Publisher | View at Google Scholar -
The Ayurvedic Pharmacopoeia of India (2016). PCIM&H, Ministry of AYUSH, Government of India. 1st ed., Vol-IX, p. 93-94.
View at Publisher | View at Google Scholar -
United States Pharmacopoeia (USP). Available from
View at Publisher | View at Google Scholar -
Martindale, The Extra Pharmacopoeia, 31st ed., Royal Pharmaceutical Society, London, 1996, p.1240.
View at Publisher | View at Google Scholar -
WHO monographs on selected medicinal plants, Vol. 1, World Health Organization, Geneva, Switzerland, 1999, p. 241-258.
View at Publisher | View at Google Scholar -
Guidance Manual for Monographs Development of Herbs and Herbal Products Including Phytopharmaceutical Drugs. 2016. The Indian Pharmacopoeia Commission, Ghaziabad. Available from
View at Publisher | View at Google Scholar -
Vander Heyden, Y., Nijhuis, A., Smeyers-Verbeke, J., Vandeginste, B.G.M. and Massart, D.L., (2001). Guidance for robustness/ruggedness tests in method validation. Journal of pharmaceutical and biomedical analysis, 24(5-6), pp.723-753.
View at Publisher | View at Google Scholar -
Ferreira, S.L., Caires, A.O., Borges, T.D.S., Lima, A.M., Silva, L.O. and dos Santos, W.N., (2017). Robustness evaluation in analytical methods optimized using experimental designs. Microchemical Journal, 131, pp.163-169.
View at Publisher | View at Google Scholar -
Validation of Analytical Procedures Q2 (R2), (2022). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. ICH Harmonised Guideline.
View at Publisher | View at Google Scholar -
Tan, P., Zhang, L., Zhao, Y.L., Zhang, C.E., Niu, M., Xiao, X.H. and Wang, J.B., (2016). A practical method for the simultaneous quantitative determination of twelve anthraquinone derivatives in rhubarb by a single-marker based on ultra-performance liquid chromatography and chemometric analysis. Analytical Methods, 8(19), pp.3927-3934.
View at Publisher | View at Google Scholar -
Fu, Y. and Kao, W.J., (2010). Drug release kinetics and transport mechanisms of non-degradable and degradable polymeric delivery systems. Expert opinion on drug delivery, 7(4), pp.429-444.
View at Publisher | View at Google Scholar -
Khan, K.A., Khan, G.M., R ehman, A.U. and Shah, K.U., (2013). Studies on Drug Release Kinetics of Controlled Release Matrices of Flurbiprofen and Comparison with Market Product. LATIN AMERICAN JOURNAL OF PHARMACY, 32(9), pp.1321-1328.
View at Publisher | View at Google Scholar -
Khatri, R., Hooda, T., Gupta, R., Kumar, P. and Jawal, P., (2018). Release kinetic study of enteric coating of senna tablet. Pharmaspire, 10, pp.29-40.
View at Publisher | View at Google Scholar -
Sun, S.W. and Su, H.T., (2002). Validated HPLC method for determination of sennosides A and B in senna tablets. Journal of pharmaceutical and biomedical analysis, 29(5), pp.881-894.
View at Publisher | View at Google Scholar -
Shriniwas S., Revanasiddappa M., World Journal of Pharmaceutical Research, volume 4, Development and validation of a stability-indicating RP-HPLC method for assay determination of methylparaben, propylparaben, potassium sorbate and sennosides, p. 1521-1544.
View at Publisher | View at Google Scholar -
Ghassemi-Dehkordi, N., Ghanadian, M. and Arabha, S., (2014). Development of a validated HPLC method for the determination of sennoside A and B, two major constituents of Cassia obovata Coll. Journal of HerbMed Pharmacology, 3(2), pp.119-124.
View at Publisher | View at Google Scholar -
Panichayupakaranant, P., Sakunpak, A. and Sakunphueak, A., (2009). Quantitative HPLC determination and extraction of anthraquinones in Senna alata leaves. Journal of chromatographic science, 47(3),pp.197-200.
View at Publisher | View at Google Scholar






